 Image Source: https://systems.jhu.edu/research/public-health/ncov/
Image Source: https://systems.jhu.edu/research/public-health/ncov/
COVID-19’s End of the Beginning
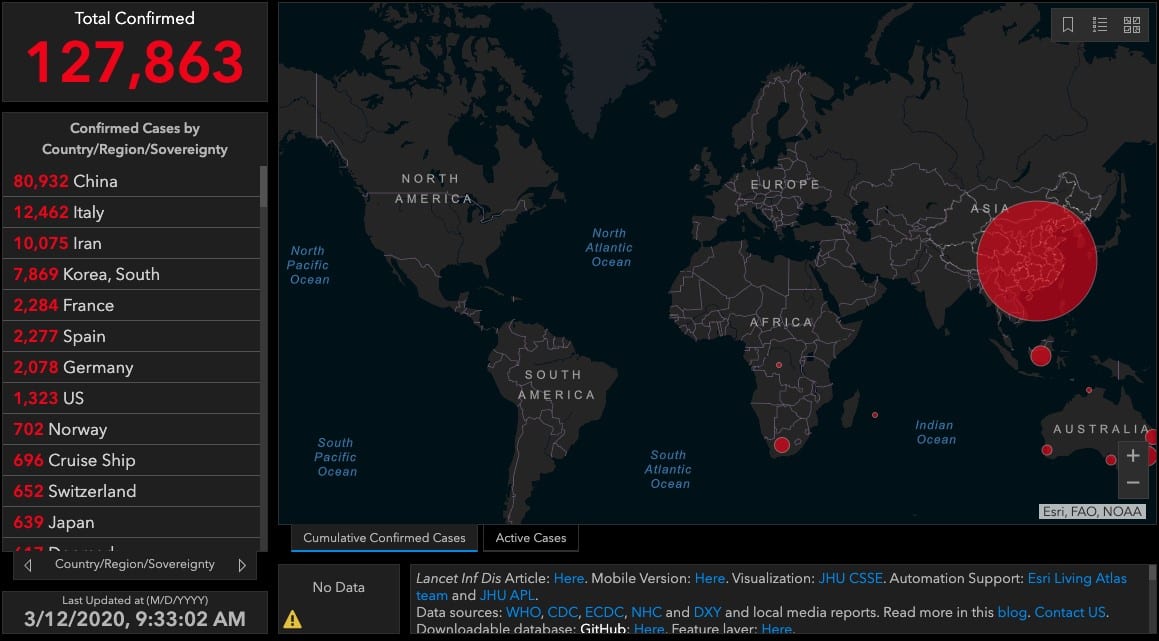
On 31-December-2019, Chinese officials alerted the World Health Organization of an outbreak of pneumonia cases in Wuhan, a city of 11-million and major hub for transportation, manufacturing, technology, and education. By 9-January, the etiologic agent was ascribed to a novel coronavirus (COVID-19), the same class of pathogen known to cause other infamous severe respiratory diseases: SARS and MERS. COVID-19 since spread beyond its quarantine in Wuhan to provinces beyond Hubei within 30 days, and globally to countries such as Italy, Iran, Korea, Spain, France, Germany, the USA, Switzerland, and Japan. As of this writing, over 120,000 people were diagnosed with the virus, and there are over 4,000 attributed fatalities.[link to real time map, 1] A geometric rate of expansion is observed in cases detected outside of China, now emerging as a world pandemic to impact with major strain on health systems, international and regional supply networks, and the labors to combat the epidemic, itself. The abrupt appearance, prolonged incubation period, and swift transmission of COVID-19 thus underscores a worldwide imperative[2] to characterize,[3, 4, 5] prevent,[6] detect,[7, 8] monitor,[1, 9] treat,[10, 11] and rehabilitate this disease[12, 13] or similar ones.[14, 15, 16] With insufficient data to model how immunity builds after a primary COVID-19 epidemic, it is difficult to predict whether these outbreaks will recur in waves, as was observed a century ago with H1N1 flu.
Most people diagnosed with COVID infection recover after a mild disease with flu-like symptoms. Nevertheless, about 14% of patients with COVID-19 infections develop breathing issues and other severe complications, and 6.1% require intensive critical care due to lung failure, septic shock, and multiple organ failure.[17] Autopsies of patients who succumbed suggest that damage to lungs from both infection and cytokine storm could yield permanent, irreversible fibrosis; pulmonary alveoli and cilia may be left unrepaired due to critical loss of respiratory tract stem cells. Of patients who died, time from initial symptoms to death can range between 2-8 weeks. On average, recovery from severe disease may take 3-6 weeks. In turn, a damaged patient’s lungs could potentially serve as a fatal incubator for secondary, drug-resistant nosocomial infections that are prevalent in hospitals and nursing homes, or among the immunocompromised. Under recent circumstances where severe COVID-19 cases appear to double every six days, impact on vital public services such as education, emergency medical services (EMS), and first responders is already non-trivial, all while finite health resources for yesterday’s routine surgeries are reallocated towards emergency footing on-the-spot. In addition to effects on the overall population and civilian workforce, there are implications for US military combat and disaster relief readiness. One critical concern involves both training and wartime operations. These are frequently conducted in close quarters, e.g., Naval ships. Both COVID-19 itself and post-infection damage could greatly impact fitness for duty, force readiness, and ultimately, possible premature exit of service for our Warfighters.
Enter MSCs into the Solutions Set Against COVID-19
Efforts to treat damage to lungs or facilitate rehabilitation in COVID-19 may reap plausible benefit via allogeneic mesenchymal stem/stromal cells (MSCs), which have been deployed into tens of thousands of patients via over 1000 unique clinical trials in the last 10 years, including several for non-COVID related respiratory distress.[18] In large animal preclinical or clinical applications, MSCs or their secreted products (e.g. paracrine factors, exosomes, or mitochondria)[19] can improve oxygenation, and reduce extravascular lung fluid, pulmonary edema, and vascular permeability.[13, 14, 15, 16, 20, 21] There are also anti-inflammatory and antimicrobial effects observed. MSCs from qualified donors have exhibited an outstanding safety record in a wide range of clinical trials, spanning a large number of patients and delivery modalities. These exogenously delivered cells then have been consistently shown to home toward zones of injury, including the lung, where they can mitigate the progression of damage or potentially even restore or repair cellular functions. The sudden, bilateral damage to lungs via coronavirus and its specter of permanent damage to the resident stem cells and endogenous regeneration capacity has been noted by infectious disease experts such Dr. Jeffery Taubenberger of NIAID in a recent press article. Therefore, MSC regenerative therapy may be a potentially effective means to pivot the patient’s outcome during the disease’s progression through critical care and/or rehabilitation.
Out of 59 directly interventional human clinical trials underway queried by clinicaltrials.gov, at least 7 involve MSCs. [22-28] Out of at least 437 coronavirus-related trials listed by the WHO’s international clinal trials registry search portal, 18 MSC-related trials were also identified.[29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46] Although these are all located in hardest-hit China, recent announcements by Mesoblast and others to evaluate their allogeneic manufactured MSC products for human COVID-19 imply that this therapeutic modality will find broad global appeal.
Taking Stock of Today’s MSC Manufacturing and the Impact on a Rapid Pandemic Response
Wallstreet likes to playfully say that Regenerative Medicine has 3 major issues: Manufacturing, Manufacturing and Manufacturing. While manufacturing definitely remains a major problem for autologous CAR-T therapies, there are also significant, but not insurmountable manufacturing challenges, relating to allogeneic hMSC therapies that could hamper a rapid disaster response for a pandemic treatment.
Let’s ask ourselves, as an industry, whether today’s therapeutic hMSC Manufacturing Readiness can now supply thousands of patient doses of therapeutic-grade, cGMP manufactured MSCs for rapid deployment by the end of 2020? The answer is unfortunately a resounding “No.” Most late-stage hMSC production processes still involve manufacture via 10-layer vessels with heavily constrained scalability (see Rowley, et al. article in Bioprocess International).[47, 48, 49] Today’s manufacturing processes can yield only 10s of product doses per manufacturing lot, severely limiting a possible rapid deployment of product doses.
Yet, is our industry getting closer to scalable production methodologies? The authors of this blog would like to optimistically state that “Yes!” A bolus of private/ funding of scalable manufacturing platforms for hMSCs facilitated a significant amount of work that can be directly leveraged. An additional bolus of funds can get us over the finish line of rapidly deployed therapeutic solutions. If the right resources are put behind the right initiatives, these scalable manufacturing approaches will be “force multipliers” against the global pandemic and help to assure that sufficient product can be generated to meet the scale requirements that are upon us today. Let’s dig into some of the numbers that will drive continued investment in large-scale MSC manufacturing sciences.
Defining Target Requirements, Engineering for Scale
Here, we calculate some back-of-envelope estimates for manufacturing scale requirements and lot sizes for rapid deployment of hMSCs to treat and rehabilitate COVID-19 related lung disorders. We will start with a few assumptions:
- Let’s assume that we need to rapidly deploy hMSC doses for 10,000 patients in a short time.
- Support: News articles (via Bloomberg) based on WHO data[17] state that 10-15% of mild cases of COVID-19 progress towards severe cases. Thus, if 75,000 patients contract COVID-19 in winter 2020 (an average caseload of severe flu in the USA alone), one could expect 7,500-11,000 patients to be at risk of significant lung injury due to COVID-19 infection. We would thus expect there to be a conservative need for 10,000 patients to be treated in a single season in the USA, which accounts for a mere 1/25th of the world’s population.
- Let’s assume by rounding, an average cell dose of 300 million (M) cells per patient, based on an average total (outliers excluded) of 316M MSC per patient from available trial data.
- This estimate comes from an analysis of several clinical trials where hMSCs were used to treat ARDS. See table below:
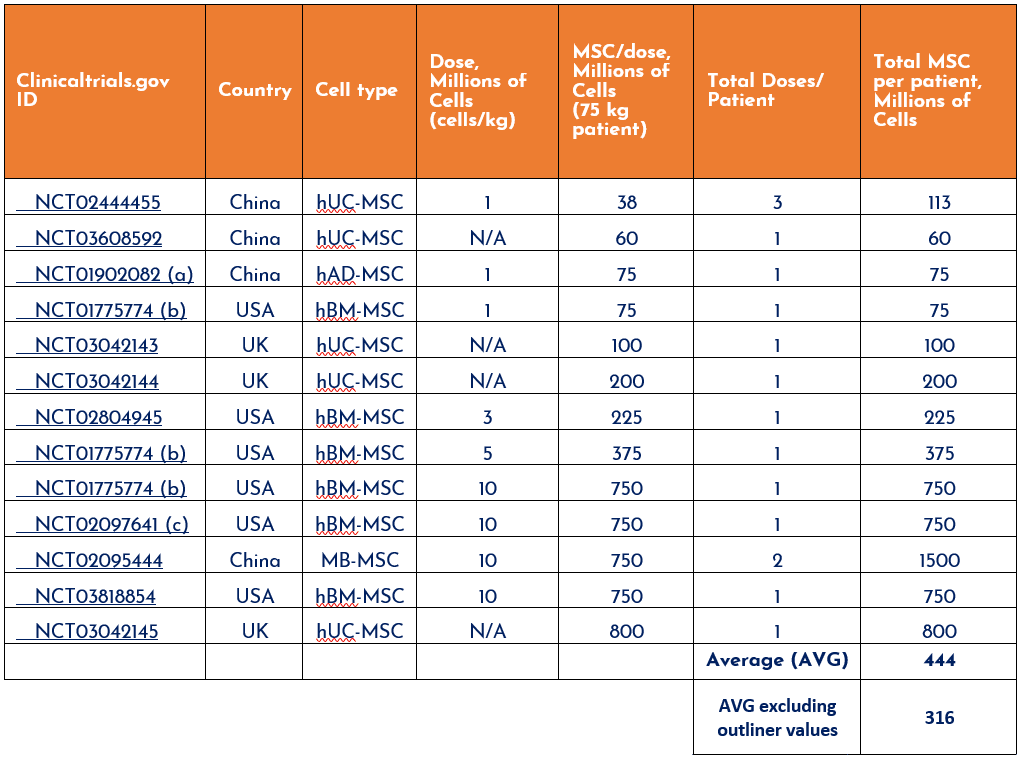
Table 1. Raw data from Cruz and Rocco, J Thorac Dis 2019;11(Suppl 9):S1329-S1332.
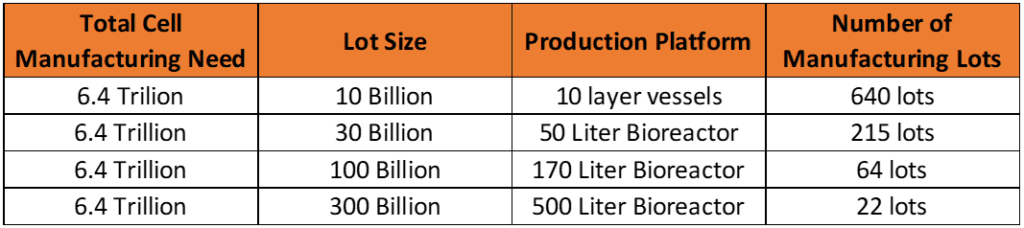
- We estimate around 6.4 Trillion cells to be manufactured to supply sufficient therapeutic product for 10,000 patients at a dose of 300M cells/patient.
-
- We used assumptions from a prior blog post on how to estimate hMSC manufacturing lot size. Given that cell therapies are typically over-filled by 50% to account for loss during cryopreservation, 450M cells would then be required per patient, multiplied by 10,000 patients. Further, accounting for 30% product loss during downstream purification and 10% loss of product due to QC testing, a total of around 6.4 x 1012, or 6.4 trillion vialed cells could be an attainable manufacturing goal. This isn’t even considering other manufacturing loses (i.e. scrapped lots)—or overproducing doses that inventory could be held at multiple different locations around the US. We also don’t reckon for any activity outside of the USA, which could expand into a cell quantity 25-fold larger in number, or 164 trillion.
-
- Making these 6.4 trillion cells via 10 billion-cell manufacturing lots would require a total of 640 manufacturing runs. This might be possible, but with CAR-T cells siphoning most reserve biomanufacturing capacity today, it’s unlikely that such manufacturing capacity for 10-layer production of hMSCs exists in the field.
-
- Scaling up production of 6.4 trillion cells to 100B cell manufacturing lots would require 64 manufacturing lots – a much more reasonable number, but still a formidable expense of surplus cGMP bioreactor capacity, clinical grade media, and testing per lot.
-
- In contrast, 6.4 trillion cells produced via 300B cell manufacturing lots, potentially in a 500L bioreactor, would require only ~21 manufacturing lots – a reasonable manufacturing pace to have a reasonably priced and consistent supply. This is therefore our recommendation as a target lot size for rapid deployment.
A Silver Lining…
Scalable bioprocess such as this isn’t merely gedankenexperiment. With a prescient eye toward crises such as today’s unfolding pandemic, the “good news” is that this type of work has been proactively funded by organizations such as the Medical Technology Enterprise Consortium (MTEC). MTEC’s first funding cycle was focused on Regenerative Medicine Manufacturing, enabling a collaboration led by BioBridge Global that included RoosterBio, the US Army Institute for Surgical Research, and StemBioSys to create a xeno-free 50L bioreactor manufacturing process (see the technical poster of this effort) for hMSCs,[50] scalable to several-100 liter volumes. Feasibility studies at the 50L scale clearly highlight a direct path towards scale-up to ~500L, to achieve a target 300B hMSC lot size, with associated downstream processing technology developed with integrated systems that can function with these production capacities as well.
The Call to Action
To expedite emerging cellular and regenerative therapies, the goals of the 21st Century Cures Act (link to overview) are supported by efforts to enable manufacturing process and standards development (FDA statement on Advanced Manufacturing). The ongoing mission of the Manufacturing USA and Cell Manufacturing Technologies (CMaT) and its pursuant Technology Roadmaps for scalable cell manufacturing demands an urgent call to action. Specifically, a mushrooming global and national pandemic crisis ought to impel biomanufacturing readiness levels toward a meaningful, full-spectrum pathogen response. Now that the “zero hour” is upon us, it’s worth reminding that we don’t need to build this readiness from the ground up—many in our community have been unceremoniously laboring toward the confrontation with this unfortunate turn of events for many years.
Disaster preparedness initiatives are often deprioritized, particularly in times of relative comfort and security. Yet we now face an even greater challenge, and that is the actual protection of human life in the face of actual danger. Together, let’s be ready to roll up our sleeves and do our duty!
Further Reading on hMSC Manufacturing
RoosterBio has written or contributed to blog posts or publications pertaining to:
- The Cell Manufacturing Roadmap 2017 update
- The Cell Manufacturing Roadmap to 2020
- Estimating manufacturing lot sizes based on possible future demand
- The manufacturing platforms required in order to achieve lot sizes of varying scales
- The role of manufacturing platforms on lot size and bioprocess economics, published in BioProcess International
- Review the RoosterBio Blog for other articles of interest
- Dong, E, H Du, and L Gardner, An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis, 2020. 1016/S1473-3099(20)30120-1
- Wang, C, PW Horby, FG Hayden, and GF Gao, A novel coronavirus outbreak of global health concern. Lancet, 2020. 395(10223): p. 470-473. 1016/S0140-6736(20)30185-9
- Huang, C, et al., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 2020. 395(10223): p. 497-506. 1016/S0140-6736(20)30183-5
- Walls, AC, YJ Park, MA Tortorici, A Wall, AT McGuire, and D Veesler, Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell, 2020. 1016/j.cell.2020.02.058
- Guan, WJ, et al., Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med, 2020. 1056/NEJMoa2002032
- Chen, W-H, U Strych, PJ Hotez, and ME Bottazzi, The SARS-CoV-2 Vaccine Pipeline: an Overview. Current Tropical Medicine Reports, 2020: p. 1-4. 1007/s40475-020-00201-6
- Chan, JF, CC Yip, KK To, TH Tang, SC Wong, KH Leung, AY Fung, AC Ng, Z Zou, HW Tsoi, GK Choi, AR Tam, VC Cheng, KH Chan, OT Tsang, and KY Yuen, Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/Hel real-time reverse transcription-polymerase chain reaction assay validated in vitro and with clinical specimens. J Clin Microbiol, 2020. 1128/JCM.00310-20
- Zhang, Y, N Odiwuor, J Xiong, L Sun, RO Nyaruaba, H Wei, and NA Tanner, Rapid Molecular Detection of SARS-CoV-2 (COVID-19) Virus RNA Using Colorimetric LAMP. medRxiv, 2020: p. 2020.02.26.20028373. 1101/2020.02.26.20028373
- Kilianski, A, et al., org: pathogen detection and characterization through a web-based, open source informatics platform. BMC Bioinformatics, 2015. 16: p. 416. 10.1186/s12859-015-0840-5
- Li, G and E De Clercq, Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat Rev Drug Discov, 2020. 19(3): p. 149-150. 1038/d41573-020-00016-0
- Zhu, R-f, R-l Gao, S-H Robert, J-p Gao, S-g Yang, and C Zhu, Systematic Review of the Registered Clinical Trials of Coronavirus Diseases 2019 (COVID-19). medRxiv, 2020: p. 2020.03.01.20029611. 1101/2020.03.01.20029611
- Chen, J, C Hu, L Chen, L Tang, Y Zhu, X Xu, L Chen, H Gao, X Lu, L Yu, X Dai, C Xiang, and L Li, Clinical study of mesenchymal stem cell treating acute respiratory distress syndrome induced by epidemic Influenza A (H7N9) infection, a hint for COVID-19 treatment. Engineering, 2020. 1016/j.eng.2020.02.006
- Liu, A, X Zhang, H He, L Zhou, Y Naito, S Sugita, and JW Lee, Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin Biol Ther, 2020. 20(2): p. 125-140. 1080/14712598.2020.1689954
- ‘Mahida, RY, S Matsumoto, and MA Matthay, Extracellular Vesicles: A New Frontier for Research in Acute Respiratory Distress Syndrome. Am J Respir Cell Mol Biol, 2020. 1165/rcmb.2019-0447TR
- Matthay, MA, et al., Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med, 2019. 7(2): p. 154-162. 1016/S2213-2600(18)30418-1
- Abraham, A and A Krasnodembskaya, Mesenchymal stem cell-derived extracellular vesicles for the treatment of acute respiratory distress syndrome. Stem Cells Transl Med, 2020. 9(1): p. 28-38. 1002/sctm.19-0205
- Organization, World Health Organization, Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). 2020.
- Cell Trials Data. Available from: https://celltrials.org/cells-data.
- Caplan, AI and D Correa, The MSC: an injury drugstore. Cell Stem Cell, 2011. 9(1): p. 11-5. 1016/j.stem.2011.06.008
- Asmussen, S, H Ito, DL Traber, JW Lee, RA Cox, HK Hawkins, DF McAuley, DH McKenna, LD Traber, H Zhuo, J Wilson, DN Herndon, DS Prough, KD Liu, MA Matthay, and P Enkhbaatar, Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax, 2014. 69(9): p. 819-25. 1136/thoraxjnl-2013-204980
- Zikuan Leng, RZ, Wei Hou, Yingmei Feng, Yanlei Yang, Qin Han, Guangliang Shan, Fanyan Meng, Dongshu Du, Shihua Wang, Junfen Fan, Wenjing Wang, Luchan Deng, Hongbo Shi, Hongjun Li, Zhongjie Hu, Fengchun Zhang, Jinming Gao, Hongjian Liu, Xiaoxia Li, Yangyang Zhao, Kan Yin, Xijing He, Zhengchao Gao, Yibin Wang, Bo Yang, Ronghua Jin, Ilia Stambler, Lee Wei Lim, Huanxing Su, Alexey Moskalev, Antonio Cano, Sasanka Chakrabarti, Kyung-Jin Min, Georgina Ellison-Hughes, Calogero Caruso, Kunlin Jin, Robert Chunhua Zhao, Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging and disease: p. 216-228. 14336/ad.2020.0228
- Hospital, B, V CELL, L GENE ENGINEERING CORP., China, W Wuhan Huoshenshan Hospital, China, TH Hospital, STPs Hospital, SY-SU Fifth Affiliated Hospital, C Wuhan Union Hospital, and WC Hospital, Treatment With Mesenchymal Stem Cells for Severe Corona Virus Disease 2019(COVID-19). 2020, https://ClinicalTrials.gov/show/NCT04288102.
- Hospital, R, SPHC Center, W Wuhan Jinyintan Hospital, China, and CBG Ltd., A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia. 2020, https://ClinicalTrials.gov/show/NCT04276987.
- Hospital, B, H Innovative Precision Medicine Group , China., W Wuhan Huoshenshan Hospital, China, TH Hospital, V CELL, L GENE ENGINEERING CORP., China, STPs Hospital, and SY-SU Fifth Affiliated Hospital, Mesenchymal Stem Cell Treatment for Pneumonia Patients Infected With 2019 Novel Coronavirus. 2020, https://ClinicalTrials.gov/show/NCT04252118.
- Wuhan Union Hospital, C and L Wuhan Hamilton Bio-technology Co., China., Study of Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Novel Coronavirus Severe Pneumonia. 2020, https://ClinicalTrials.gov/show/NCT04273646.
- CAR-T Biotechnology Co., L, Novel Coronavirus Induced Severe Pneumonia Treated by Dental Pulp Mesenchymal Stem Cells. 2020, https://ClinicalTrials.gov/show/NCT04302519.
- Science, PHAtWUo, Technology, and L Wuhan Hamilton Bio-technology Co., Therapy for Pneumonia Patients iInfected by 2019 Novel Coronavirus. 2020, https://ClinicalTrials.gov/show/NCT04293692.
- Peng, Z, TBTC Ltd, and Z Hospital, Umbilical Cord(UC)-Derived Mesenchymal Stem Cells(MSCs) Treatment for the 2019-novel Coronavirus(nCOV) Pneumonia. 2020, https://ClinicalTrials.gov/show/NCT04269525.
- ChiCTR2000029569, Safety and efficacy of umbilical cord blood mononuclear cells conditioned medium in the treatment of severe and critically novel coronavirus pneumonia (COVID-19): a randomized controlled trial. 2020.
- ChiCTR2000029580, Severe novel coronavirus pneumonia (COVID-19) patients treated with ruxolitinib in combination with mesenchymal stem cells: a prospective, single blind, randomized controlled clinical trial. 2020.
- ChiCTR2000029816, Clinical Study for Cord Blood Mesenchymal Stem Cells in the Treatment of Acute Novel Coronavirus Pneumonia (COVID-19). 2020.
- ChiCTR2000029817, Clinical Study of Cord Blood NK Cells Combined with Cord Blood Mesenchymal Stem Cells in the Treatment of Acute Novel Coronavirus Pneumonia (COVID-19). 2020.
- ChiCTR2000029990, Clinical trials of mesenchymal stem cells for the treatment of pneumonitis caused by novel coronavirus pneumonia (COVID-19). 2020.
- ChiCTR2000030020, The clinical application and basic research related to mesenchymal stem cells to treat novel coronavirus pneumonia (COVID-19). 2020.
- ChiCTR2000030088, Umbilical cord Wharton’s Jelly derived mesenchymal stem cells in the treatment of severe novel coronavirus pneumonia (COVID-19). 2020.
- ChiCTR2000030116, Safety and effectiveness of human umbilical cord mesenchymal stem cells in the treatment of acute respiratory distress syndrome of severe novel coronavirus pneumonia (COVID-19). 2020.
- ChiCTR2000030138, Clinical Trial for Human Mesenchymal Stem Cells in the Treatment of Severe Novel Coronavirus Pneumonia (COVID-19). 2020.
- ChiCTR2000030173, Key techniques of umbilical cord mesenchymal stem cells for the treatment of novel coronavirus pneumonia (COVID-19) and clinical application demonstration. 2020.
- ChiCTR2000030224, Clinical study of mesenchymal stem cells in treating severe novel coronavirus pneumonia (COVID-19). 2020.
- ChiCTR2000030261, A study for the key technology of mesenchymal stem cells exosomes atomization in the treatment of novel coronavirus pneumonia (COVID-19). 2020.
- ChiCTR2000030300, Umbilical cord mesenchymal stem cells (hucMSCs) in the treatment of high risk novel coronavirus pneumonia (COVID-19) patients. 2020.
- NCT04252118, Mesenchymal Stem Cell Treatment for Pneumonia Patients Infected With 2019 Novel Coronavirus. 2020.
- NCT04269525, Umbilical Cord(UC)-Derived Mesenchymal Stem Cells(MSCs) Treatment for the 2019-novel Coronavirus(nCOV) Pneumonia. 2020.
- NCT04273646, Study of Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Novel Coronavirus Severe Pneumonia. 2020.
- NCT04276987, A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia. 2020.
- NCT04293692, Therapy for Pneumonia Patients iInfected by 2019 Novel Coronavirus. 2020.
- Rowley, J, E Abraham, A Campbell, H Brandwein, and S Oh, Meeting lot-size challenges of manufacturing adherent cells for therapy. BioProcess Int, 2012. 10(3): p. 7.
- Christy, BA, MC Herzig, CP Delavan, R Kirian, T Olsen, T Ahsan, AP Cap, J Rowley, and JA Bynum. Mesenchymal Stem Cells Grown in a Bioreactor Are Functionally Similar to Those Grown in Monolayer Culture. in 2019 Annual Meeting. 2019. AABB.
- Lembong, J, D Wang, R Kirian, A-C Tsai, K Cruz, F Rosello, K Cox, Y Hashimura, S Jung, and T Ahsan, A scalable xeno-free microcarrier suspension bioreactor system for regenerative medicine biomanufacturing of hMSCs.
- R. Kirian, D. Wang, J. Takacs, A. Tsai, K. Cruz, F. Rosello, K. Cox, Y. Hashimura, J. Lembong and J. Rowley. Cytotherapy. SCALING A XENO-FREE FED-BATCH MICROCARRIER SUSPENSION BIOREACTOR SYSTEM FROM DEVELOPMENT TO PRODUCTION SCALE FOR MANUFACTURING XF hMSCs. 2019.
