Listen to this Blog:
A Brief History of Cell-Derived Particles
Extracellular Vesicle (EV)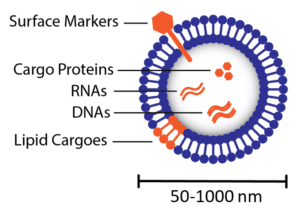
Figure 1. Illustration of an extracellular vesicle. RNAs = ribonucleic acids; DNAs = deoxyribonucleic acids. |
Tiny particles, invisible to the naked eye, were first described being ‘jettisoned’ from red blood cells when observed using an electron microscopy technique in the 1980s [1, 2]. At the time, most researchers regarded these particles as mere waste products, and thus they were referred to commonly as ‘cell dust’ or ‘cell debris’. However, over time, the abundant presence of these nano-sized particles was continually re-discovered when analyzing biological fluids, prompting some researchers to posit that they may serve a biological purpose. Composed of a lipid bilayer like their parent cells, it stood to reason that these tiny particles could be involved in communications between cells.
To first investigate this new idea, in 1996, Raposo et al. showed that isolated particles secreted by B cells could be delivered to T cells in order to make them proliferate [3]. This was the first demonstration that cell-derived particles alone could modify cellular behaviors. In 2006, Ratajczak et al. confirmed that cell-derived particles can modify recipient cell behavior and further demonstrated that these particles contain ribonucleic acids (RNAs) and proteins, which they suggest are delivered to cells through the particles [4]. Valadi et al. followed up on this result in 2007 by isolating particles secreted by mast cells, and showed that they contained over 1000 distinct RNA transcripts including messenger RNAs (mRNAs) and micro RNAs (miRNAs) [5]. These studies collectively demonstrated that cell-derived particles contain biological contents and, in turn, transfer such content between cells, which can lead to changes in recipient cell behavior. This affirms their importance for not just waste disposal, but also for intercellular communication.
What are Extracellular Vesicles & Exosomes?
Today, cell-derived particles are conventionally referred to by the name extracellular vesicles (or EVs for short) and are defined as lipid bilayers 50-1000 nm in diameter, produced by cells for intercellular communications such as signaling, immune modulation, or molecular recycling [6]. Because of their extremely small size, extracellular vesicles cannot be visualized using light microscopy. Instead, beginning in earnest with a seminal work by Sokolova et al in 2011, researchers use techniques such as electron microscopy to directly visualize particles, or dynamic light scattering to indirectly visualize particles [7].
Extracellular vesicles can be classified into subgroups that are defined by their subcellular compartment of origin. Exosomes are EVs typically described as smaller in size, ranging from 40-160nm in diameter [6]. They form by budding inward within endosomes in cells, leading to formation of multivesicular endosomes (MVEs), defined simply as endosomes that contains many exosomes [8]. To release exosomes into the extracellular space, MVEs fuse with the plasma membrane. Microvesicles, or ectosomes, are extracellular vesicles comprising a broader size range, ranging from 50-1000nm in diameter [6], and form by direct budding at the plasma membrane leading to direct release into the extracellular space [8]. Also considered extracellular vesicles, apoptotic bodies bud directly from the plasma membrane while cells undergo apoptosis, or programmed cell death, but they tend to be larger particles (generally described as ≥500nm in diameter [9]). Since all extracellular vesicles are derived from the cellular membrane, their own membranes contain cellular membrane components such as transmembrane proteins. EV membranes are known to be enriched in tetraspanins [6], a type of transmembrane protein, and thus EVs are typically identified by the presence of these ‘surface markers’ on the particles (Figure 1), namely the tetraspanins CD63, CD9, and CD81 [10]. Extracellular vesicles contain not only RNAs and proteins, but also can contain DNAs [11] and lipids [12] as biological cargoes to transfer to recipient cells.
Becoming an Independent Therapeutic Agent
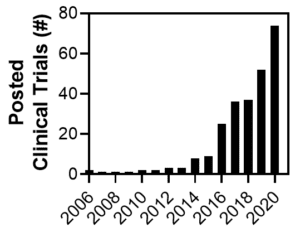
Figure 2. Number of clinical trials investigating EVs as therapeutic agents or biological readout material. Obtained from clinicaltrials.gov with query, other terms: “extracellular vesicles” OR “exosomes” first posted within given year. Search on 17Jun2021. |
As cell therapy emerged as a preeminent new category of medicine, particularly for mesenchymal stromal cells (MSCs), many within the field realized that the medium used to culture cells (conditioned medium) was potentially sufficient to observe therapeutic effects [13, 14]. For example, in 2011 Timmers et al showed that conditioned medium from human MSCs improves recovery after heart attack in pigs [15]. Over the same timeframe, the discovery of extracellular vesicles as a communication medium between cells led researchers to posit that EVs produced by cells may have natural therapeutic potential, since EVs can reprogram recipient cell behavior through cargo delivery as described above. In a couple of landmark studies, the researchers showed that treating mice with EVs derived from mesenchymal stromal cells (MSCs) grant therapeutic efficacy in models of acute kidney injury [16] and myocardial ischemia/reperfusion injury [17]. Since then, the therapeutic capabilities of EVs expanded to include several other diseases and modes of action, because researchers recognized that therapeutic cargoes can exist naturally in the EVs or they can be introduced by a variety of other methods [18].
To date, the largest grouping of preclinical and clinical studies involving therapeutic extracellular vesicles has focused on EVs derived from MSCs [19]. Over the past decade, MSC-EVs have proven to show innate therapeutic capabilities with great promise as treatments against a range of over 15 different preclinical disease indications, including liver, heart, kidney, neurodegenerative, and lung disease [20]. Preclinical studies showing therapeutic efficacy of MSC-EVs are typically performed in rodents, and efficacy is attributed to the protein and/or RNA cargoes delivered by EVs [21]. The first study involving MSC-EV treatment in a human patient was reported in 2014 for a patient with untreatable graft versus host disease (GvHD) [22]. The patient suffered no side effects from treatment and multiple disease symptoms improved significantly. Since then, several other clinical trials involving MSC-EVs have been completed or are ongoing for disease indications including macular degeneration, type 1 diabetes, and chronic kidney disease (for more information, see Wiklander et al. [19] and Maumus et al. [23]). Furthermore, efforts have been made to demonstrate therapeutic efficacy in human patient samples, such as Silva et al recently demonstrated by applying MSC-EVs to precision-cut lung samples from patients with acute respiratory distress syndrome [24]. These efforts will profoundly impact the rate by which MSC-EV therapies reach the clinical setting.
Challenges & Solutions on the Road to the Clinic
Promising results described above have led to an exponential increase in the total number of clinical trials investigating extracellular vesicles either as direct therapeutic agents or as biomarker readout material from 2006 to 2020 (Figure 2). With this large number of clinical trials, one may wonder how many extracellular vesicles are required to achieve a significant therapeutic dose [25] and whether this dose varies across disease indications or administration routes. Though leaders in the field have banded together to attempt to standardize the definition of therapeutic extracellular vesicles [26], MSC-EV dosing standards remain largely non-standardized, and these considerations will be discussed in an upcoming blog post. Furthermore, what technologies will make it possible to produce enough extracellular vesicles to support the large number of upcoming clinical trials [27, 28]? To meet these challenges, RoosterBio has established products and data-driven processes that yield high-quality MSCs under xeno-free conditions at a large scale [29]. Another future blog post will present and discuss the strategies and solutions in development at RoosterBio to address MSC-EV production bottlenecks.
References
- Pan, B. T. and R. M. Johnstone, Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell, 1983. 33(3): p. 967-78. 10.1016/0092-8674(83)90040-5
- Harding, C., J. Heuser, and P. Stahl, Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol, 1983. 97(2): p. 329-39. 10.1083/jcb.97.2.329
- Raposo, G., et al., B lymphocytes secrete antigen-presenting vesicles. J Exp Med, 1996. 183(3): p. 1161-72. 10.1084/jem.183.3.1161
- Ratajczak, J., et al., Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia, 2006. 20(5): p. 847-56. 10.1038/sj.leu.2404132
- Valadi, H., et al., Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol, 2007. 9(6): p. 654-9. 10.1038/ncb1596
- Kalluri, R. and V. S. LeBleu, The biology, function, and biomedical applications of exosomes. Science, 2020. 367(6478). 10.1126/science.aau6977
- Sokolova, Viktoriya, et al., Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids and Surfaces B: Biointerfaces, 2011. 87(1): p. 146-150. https://doi.org/10.1016/j.colsurfb.2011.05.013
- van Niel, G., G. D’Angelo, and G. Raposo, Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol, 2018. 19(4): p. 213-228. 10.1038/nrm.2017.125
- Caruso, S. and I. K. H. Poon, Apoptotic Cell-Derived Extracellular Vesicles: More Than Just Debris. Front Immunol, 2018. 9: p. 1486. 10.3389/fimmu.2018.01486
- Thery, C., et al., Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles, 2018. 7(1): p. 1535750. 10.1080/20013078.2018.1535750
- Malkin, E. Z. and S. V. Bratman, Bioactive DNA from extracellular vesicles and particles. Cell Death Dis, 2020. 11(7): p. 584. 10.1038/s41419-020-02803-4
- Skotland, T., et al., An emerging focus on lipids in extracellular vesicles. Adv Drug Deliv Rev, 2020. 159: p. 308-321. 10.1016/j.addr.2020.03.002
- Iso, Y., et al., Multipotent human stromal cells improve cardiac function after myocardial infarction in mice without long-term engraftment. Biochem Biophys Res Commun, 2007. 354(3): p. 700-6. 10.1016/j.bbrc.2007.01.045
- Dai, W., S. L. Hale, and R. A. Kloner, Role of a paracrine action of mesenchymal stem cells in the improvement of left ventricular function after coronary artery occlusion in rats. Regen Med, 2007. 2(1): p. 63-8. 10.2217/17460751.2.1.63
- Timmers, L., et al., Human mesenchymal stem cell-conditioned medium improves cardiac function following myocardial infarction. Stem Cell Res, 2011. 6(3): p. 206-14. 10.1016/j.scr.2011.01.001
- Bruno, S., et al., Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol, 2009. 20(5): p. 1053-67. 10.1681/ASN.2008070798
- Lai, R. C., et al., Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res, 2010. 4(3): p. 214-22. 10.1016/j.scr.2009.12.003
- S, E. L. Andaloussi, et al., Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov, 2013. 12(5): p. 347-57. 10.1038/nrd3978
- Wiklander, O. P. B., et al., Advances in therapeutic applications of extracellular vesicles. Sci Transl Med, 2019. 11(492). 10.1126/scitranslmed.aav8521
- Abreu, Soraia C., et al., Mesenchymal Stromal Cell-Derived Extracellular Vesicles in Lung Diseases: Current Status and Perspectives. Frontiers in Cell and Developmental Biology, 2021. 9(97). 10.3389/fcell.2021.600711
- Escude Martinez de Castilla, P., et al., Extracellular vesicles as a drug delivery system: A systematic review of preclinical studies. Adv Drug Deliv Rev, 2021. 175: p. 113801. 10.1016/j.addr.2021.05.011
- Kordelas, L., et al., MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia, 2014. 28(4): p. 970-3. 10.1038/leu.2014.41
- Maumus, M., et al., Mesenchymal Stem Cell-Derived Extracellular Vesicles: Opportunities and Challenges for Clinical Translation. Front Bioeng Biotechnol, 2020. 8: p. 997. 10.3389/fbioe.2020.00997
- Silva, Johnatas Dutra, et al., MSC extracellular vesicles rescue mitochondrial dysfunction and improve barrier integrity in clinically relevant models of ARDS. European Respiratory Journal, 2020: p. 2002978. 10.1183/13993003.02978-2020
- Lembong, Josephine. Productivity Metric Considerations in MSC & MSC-EV Manufacturing in Response to COVID-19. RoosterBio Blog 2020; Available from: https://www.roosterbio.com/evs-exosomes/productivity-metric-considerations-in-msc-msc-ev-manufacturing-in-response-to-covid-19/.
- Witwer, K. W., et al., Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles, 2019. 8(1): p. 1609206. 10.1080/20013078.2019.1609206
- Adlerz, K., et al., Strategies for scalable manufacturing and translation of MSC-derived extracellular vesicles. Stem Cell Res, 2020. 48: p. 101978. 10.1016/j.scr.2020.101978
- Rowley, Jon. Radically Simplifying Production of hMSC-EVs with Rapid Clinical Translation & Scale-up Potential. RoosterBio Webinar 2020; Available from: https://bit.ly/3AqZOQm.
- Katrina Adlerz, Michelle Trempel, Jamie Brennan, Jon Rowley, Taby Ahsan. MSC-EVs Manufactured in Scalable 3D Bioreactor Systems. 2019; Available from: https://www.roosterbio.com/wp-content/uploads/2019/10/2019-BPI-EV.pdf.
