
hMSC lot size estimation is crucial for creating a clinical and commercially viable biomanufacturing process. In this blog we outline a development program that could tailor accordingly to meet your final product platform needs, with target cell lot size foremost in mind.
At RoosterBio, one of our goals is to ensure processes are scalable to fit future needs—and built with a sustainable business model to commercialize your product sooner. This concept is consistent with the basic elements of skillful strategic planning:
- Understand what the future looks like and work backward
- Create a model using reasonable conservative assumptions along the way to estimate a range of needs
- Use this model to lay out how the next several years will play out, assuming successful achievement of intermediate milestones
- Create multi-year programs that are right-sized, with the right technology platform, for the stage of product/clinical development of your company
This is the type of consultation that we provide with our Process Development Services, which we offer to customers who incorporate RoosterBio hMSC cell banks and bioprocess media systems into their product and process development efforts. However, we love to share our knowledge to help cultivate our nascent Regenerative Medicine Industry as a whole, and are offering a glimpse of those services here in this blog.
Note to readers: this exercise advances the base assumption that this involves an Allogenic, Universal Donor manufacturing process, [1] and is not directly applicable to Autologous or patient-specific products. Yet, the core logic propositions would still hold when applied to autologous cells but at a much smaller scale.
Lot Size Targets Dictate the Production Platform
The goal of any process development program is to create a right-sized manufacturing process for your immediate business goals but with future scalability in mind. Understanding the future lot size requirements will help strategically align manufacturing requirements today—with commercial scalability—while laying a platform foundation to minimize comparability risks.
First Start With the End in Mind – Engage With Marketing
Get with the business team within your company (usually Strategic Marketing and/or Commercial Operations) and request a numbers-driven discussion on which of the multiple indications that a process development program should be built for. There are usually several therapeutic indications of interest, but it is important to realize that a manufacturing process for an ocular indication that has a dose of 1 million cells/dose is very different than a Crohn’s disease indication that has a dose size of greater than 1 billion cells/dose [2]. Each therapeutic indication from a company must be treated as a distinct product and target to be accompanied by a distinct process. Just because the same cell type is used in both therapeutic products does not mean they share a manufacturing process. An initial Target Product Profile (TPP) is thus critical to bring clarity to each program.
“A common pitfall that many cell therapy companies fall into is that each therapeutic indication is not addressed as a unique product with a unique manufacturing process.”
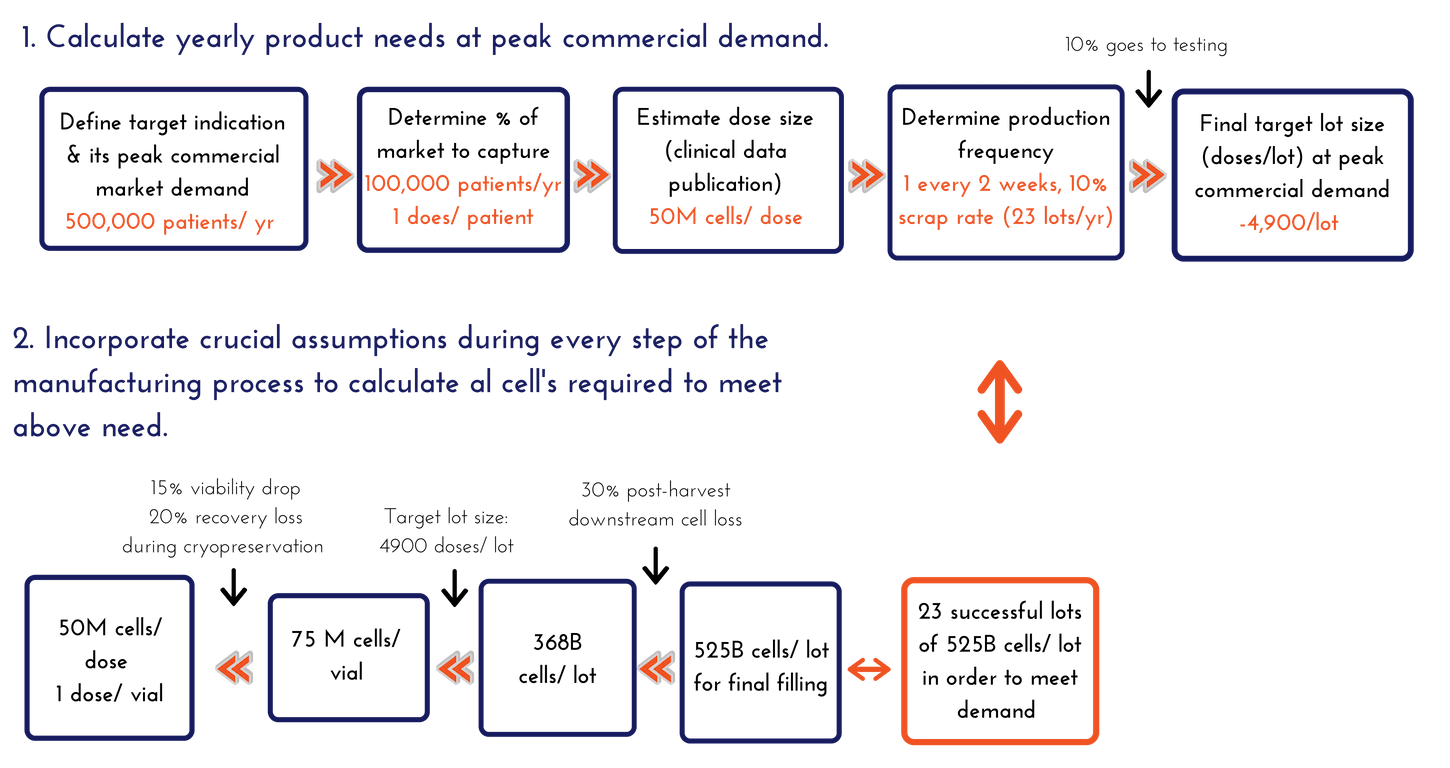
Once the team is aligned on the target indication (or set of prioritized target indications), you then need to understand what a reasonable peak commercial market demand would be. This estimate of demand likely to be found in the business model projections that are built for investors and is a good place to start. If there are 500,000 patients a year in your target indication and the business is aiming to capture 20% of them (or treat 100,000 patients/year with single doses) at peak commercial demand, then you need a process that will scale with that over time, assuming both clinical and market success. It is a good idea to establish a range. We often like to perform this exercise with low, middle, and high assumptions (such as 30,000 patients as low, 100,000 as the target, and 250,000 patients treated/year if wildly successful). For this article, we will simply focus on the 100,000 patients treated with single dose assumption for our calculations.
Dosing Assumptions: This is One of the Hardest Parts
Dose estimation is sometimes the most difficult part. Does the patient need a single dose or multiple doses and how many cells per dose? Are there published examples of the MSCs used in large animal models of the disease? In many cases, a dose-escalation trial has not yet been performed, so some educated guesstimates are required. Taking a low/mid/high range of estimates also works here, and there is sufficient published work related to hMSCs in different indications that you can get close enough for these purposes [2, 3]. For this example, we will assume a cardiac indication. There is a good amount of published work on hMSCs for cardiac indication, and we can assume a conservative low dose of 25 million cells, a mid-range dose of 50 million, and a high dose of 100 million [4, 5, 6]. In this blog post, we will perform our example calculations with an assumption of 50 million cells per dose.
Calculating Yearly Product Needs at Peak Commercial Demand
- At peak demand, we aim to produce 100,000 doses per year with a size of 50 million cells per dose
- 100,000 doses per year ≈ 2,000 doses per week
- Weekly production is not recommended – it is simply unsustainable, so let’s assume one (1) lot production every two (2) weeks, or 25 lots total in a year (which is still a lot for any manufacturing site)
- Not all lots will be successful – assuming a 10% scrap rate, 2-3 lots are lost per year, giving us 22-23 lots per year
- 100,000 doses per year / ~23 lots per year = 4,450 doses/lot
- Assume 10% of the lot goes to testing (~450 doses), a final target lot size of 4,900 doses/lot will need to be manufactured at peak commercial demand
It is important to remember that it typically takes multiple years for a successful therapeutic to reach peak demand, so it is not necessary to go to market with a manufacturing process capable of meeting peak demand – especially for an early-stage field like Regenerative Medicine. We will create estimates for peak demand but then focus on building a reasonable “go-to-market” lot size and manufacturing process.
Calculating Total Cells to Manufacture That Are Required to Fill into the Final Container
- We will assume 1 dose goes into one container.
- We assumed a mid-range dose size of 50 million (i.e. 50 million viable cells per vial post-thaw).
- Cell Count is a strict QC parameter with a straightforward specification. It is important to point out that often there is a solid safety factor, or overfill, applied to this metric. You never want to fail a lot on cell count after you spent hundreds of thousands of dollars creating the units – so the risk-averse will overfill vials until sampling and cell enumeration is an accurate, consistent, and precise method.
- Assume 15% (worst case) viability drop and 20% recovery loss during cryopreservation (these assumptions should be data-driven from your process, and constantly trended and updated during manufacturing).
- Based on the above assumptions, to consistently achieve 50 million viable cells after thaw, it is required to target filling at 75 million viable cells into the final container:
- 75 million viable cells – 15 million (20% total cell loss on recovery) = 60 million viable cells
- 60 million – 9 million (15% viability drop) = 51 million viable cell target specification
- NOTE: Some programs will only overfill by 10-20%, but we find that with this range it is very difficult to routinely obtain successful cell counts in a manufacturing/QC environment, so we recommend 30-50% overfill for calculations.
Calculating Lot Size Requirements at Peak Commercial Demand
One unfortunate fact of cell manufacturing is that you need to manufacture more cells than you need to fill, since there will be losses associated with the post-harvest processing steps. Many biological manufacturing processes have losses in the 40%-60% range in this “downstream” processing. For our process, we go through the following math:
- 75 million cells/dose × 4900 doses per manufacturing lot = 368 billion viable cells needed to target 4,900 doses from every manufacturing lot.
- We assume 30% post-harvest cell loss during processing for this exercise – thus at least 525 billion cells need to be manufactured in order to have 368 billion cells during the fill unit operation.
- 525 billion viable cells – 157 billion (30% post-harvest cell loss) = 368 billion viable cells
- 30% cell loss does seem significant, however every process development group will have a team working to optimize their process to minimize this number. It is likely that recoveries from earlier processes will be much greater than 30%, and with optimization, at large scale, it is possible to improve. For modeling, it is always worth using reasonable to conservative estimates.
In conclusion, we arrived at the following numbers:
- At peak commercial demand, 23 successful lots of 525 billion cells per lot need to be manufactured in order to meet the demand, assuming a mid-range dose for a cardiac indication aimed to treat 100,000 patients per year.
This calculation does not take into account further downstream losses associated with stability, multiple distribution sites, and safety stocks – which will just further inflate the numbers. And it does not account for any hypothetical post-thaw cell resuscitation phase to “freshen” them prior to injection. For this exercise, we will stop here. The 525 billion viable cells per lot is the number we were looking for, and it is this number that will drive the production platform decisions for streamlined hMSC clinical manufacturing.
For an early-stage RegenMed company, a go-to-market lot size of ~20% of the “peak commercial scale” is a reasonable target to plan for, so a ~100 billion cells/lot would be a good target number to develop and run your Phase III trial at.
Summary With a Few Number Recommendations:
- Phase III/Go-to-Market: ~100B cells
- Phase II (100-200 patients over 3-4 lots): 15-25B cells – this is an intermediate step up from Phase I and within a log of the Phase III/go-to-market process
- Phase I (small scale): 5-10B cells
The next step is to lay out the technology platforms for the various manufacturing unit operations capable of processing these cell numbers. This is the topic of our following blog post.
If you have an interest in discussing scalable production platforms, our complete solutions that radically simplify hMSC process development, or our Process Development Services, please reach out to us at info@roosterbio.com!
References:
- Simaria et al. (2013) Allogeneic cell therapy bioprocess economics and optimization: Single‐use cell expansion technologies. Biotechnol Bioeng 111(1): 69-83. PubMed
- Olsen et al. (2018) Peak MSC—Are We There Yet? Front Med 5:178. PubMed
- Squillaro et al. (2016) Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant 25(5):829-848. PubMed
- Majka et al. (2017) Concise Review: Mesenchymal Stem Cells in Cardiovascular Regeneration: Emerging Research Directions and Clinical Applications. Stem Cells Transl Med 6(10):1859-1867. PubMed
- Golpanian et al. (2016) Concise Review: Review and Perspective of Cell Dosage and Routes of Administration From Preclinical and Clinical Studies of Stem Cell Therapy for Heart Disease. Stem Cells Transl Med 5(2):186-191. PubMed
- Perin EC et al. (2015) A Phase II Dose-Escalation Study of Allogeneic Mesenchymal Precursor Cells in Patients With Ischemic or Nonischemic Heart Failure. Circ Res 117(6):576–584. PubMed
