
Previously, we discussed estimating hMSC lot sizes for clinical manufacturing to develop an effective multi-year process development program. This exercise is crucial, because the calculated target cell lot size dictates the final production platform needed for your therapeutic product. Our next step is to determine the appropriate manufacturing platform for each unit operation that will meet the needs of those calculated hMSC lot sizes throughout clinical development. Having a solid plan in advance will help your company succeed at navigating the complex maze that is the path to market success.
“It is the technologists’ and engineers’ jobs to drive the technology platform decision-making process.”
The final decision regarding production platforms can overwhelm. Even though there is a certain goal in mind for the present time, you do want to keep it flexible and scalable for other potential applications in the future. There is also the goal to manage the “Comparability Challenges” while these changes are implemented. Adding to the complexity is the fact that as the RegenMed industry grows, the providers of cell processing technologies across the various unit operations seem to be ramifying outwards in a divergent nature, with little standardization across the branches of competing device platforms. These technologies (e.g. bioreactors, continuous centrifuges, fill-finish/controlled freezing, and other automation platforms) are significant investments to the company in the form of cost and time. It is the technologists’ and engineers’ jobs to drive the technology platform decision making process by de-risking these technologies and establishing a multi-year development program. Meanwhile, they need to determine the costs associated with the program and communicate these needs to the company’s business team so that they can raise the needed capital for these programs over time.
The goal of this post is to lay out the various scales of hMSC production and highlight the existing technology platforms for the different unit operations involved in the manufacturing process. This will help define the requirements that will guide the company’s multi-year process development program to meet projected future lot sizes.
Each Phase of a Clinical Trial is Associated with a Specific Production Scale, Which Dictates the Production Platform
At the end of the last blog post, Building Effective Multi-Year Process Development Programs Part I, we arrived at the following estimated lot sizes based on a set of assumptions: 525 billion viable hMSCs per final commercial manufacturing lot, assuming a mid-range dose for a cardiac indication aimed to treat 100,000 patient-doses per year, with relatively safe, conservative assumptions regarding losses in cell viability and recovery during every step of the production process. Assuming a go-to-market lot size of 20% of the full commercial scale, we estimated that one could target a 100B cell lot size for Phase III, a 25B cell lot size for Phase II, and potentially a 10B cell lot size for a Phase I trial. These are simply guidelines that will change based on assumptions, but we recommend everyone go through this exercise for each therapeutic program.
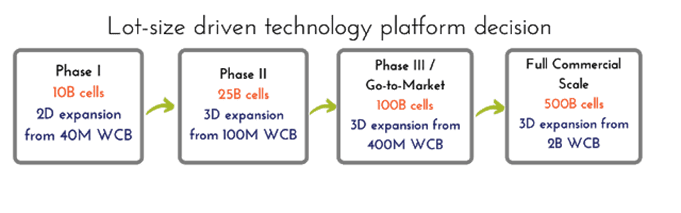
Traditional 2D hMSC manufacturing processes were capable of harvest densities of 20,000-30,000 cells/cm2 [1]. Starting with a working cell bank of 40M cells, the Phase I lot size could be achieved using 60-80 2D CellStacks at this range of harvest density. High productivity hMSC Bioprocess Systems, such as RoosterBio’s, drive cell density at harvest to ≥ 40,000 cells/cm2 while maintaining low cumulative PDL of ~16-18. The actual 2D harvest numbers can be > 60,000 cells/cm2 (this is mostly donor-dependent), however downstream processing losses typically bring the numbers back down [2], so we’d like to do all our calculations with a conservative density of 40,000 cells/cm2. This higher harvest density decreases the number of CellStacks to ~40 compared to traditional 2D planar manufacturing processes at the Phase I scale, reducing process complexity, labor, and overall Cost of Goods (COGS) significantly. Harvest processing time can be reduced by several hours and the facility footprint by hundreds of square feet, including many expensive reagents costs such as media, harvest reagent, etc. With these numbers, the processes can be developed and executed quickly in a robust 10-layer CellStack/CellFactory process, therefore getting a final hMSC product into Phase I for a proof to therapy with a low-risk process. Feasible, reproducible, streamlined, and low-cost Phase I bioprocess is usually the best path to achieve for initial pharmaceutical development, and thus drives much of the 2D platform decisions for hMSC products. We do help our customers rapidly implement these processes via our Process Development Services.
Scalable hMSC Clinical Manufacturing Needs Movement Away from 2D Planar Platforms
Each manufacturing phase that leads up to the go-to-market lot size is associated with a new scale of production, as thus requires a scale-up development program. Process development programs are time-consuming and expensive. Therefore, multi-year planning for efficient capital utilization within a company is fully justified and required. Specific time-intensive planning aspects to proactively address include budgeting for at least 6 months of disposable engineering (having each single-use bag and disposable at each unit operation with compatible connections). Next, there is planning for at least 3-6 half-scale and 3 full-scale runs to understand and de-bug a full process prior to the forthcoming tech transfer. This can easily encompass up to 12-18 months of development studies for each scale-up program.
The scale of production at our proposed Phase II lot size of 25 billion is challenging to achieve using 2D planar platforms, even via high productivity systems. Assuming a typical harvest yield of 40,000 cells/cm2, 25 billion cells in CS10’s requires ~100 CellStacks. There are several challenges with this: it is very expensive, time-consuming, labor-intensive, and not environmentally friendly (think of the >800 sq ft footprint that the CellStacks occupy and how much plastic waste that entails). The largest 2D planar platform, the automated 120-layer hyperstacks, can generate ~100B lot size; however, there is no option of scaling-up from there. At scales larger than 15-20 Billion cells/lot, we recommend moving directly to 3D bioreactor platforms to maintain rational stepwise scalability for the future. We estimate that a 100B target lot size is relevant for ~95% of the indications requiring hMSC treatment based on our analysis of cell dose from ClinicalTrials.gov [3], with some exceptions including low dose indications such as ocular diseases [4]. Therefore, we recommend a jump to a bioreactor production platform for most development programs that aim to advance into Phase II.
As the field moves into suspension bioreactors from 2D, there are new opportunities for productivity gains during production. Borrowing from best practices in the monoclonal antibody field, fed-batch processes can lead to high media productivity, and we have established this in hMSC production as well. With a fed-batch process, coupled with well QC’d, low PDL cell banks, it is routine to achieve a range of final cell yields of 0.4M – 0.8M cells/ml in a microcarrier suspension bioreactor process incorporating a bioreactor feed when performed in a low-shear Vertical-Wheel™ bioreactor. A 3D fed-batch process increases media usage efficiency by >2 times compared to our 2D batch systems (or by >15 times compared to a traditional hMSC 2D flask culture), thus simplifying the overall process, increasing cell productivity, and reducing media costs per cell produced. It is these compounded efficiencies that will drive the cell cost curve down with further scale advancements.
For bioreactor production volumes, we estimate a conservative average bioreactor yield of 0.5M cells/ml (again, this is donor-dependent, among other variables). Thus, a production vessel of 50L bioreactor is required for a 25B cell lot size. The 100B hMSC go-to-market lot size requires a 200L bioreactor, and the full-scale production requires a 1,000L bioreactor. To achieve these lot sizes while maintaining the PDL of the final product (a critical quality attribute of every hMSC final product), the working cell bank must scale in size with the process and will range from 100M to 2B (in closed, single-use formats).
Table 1. Summary of the technology landscape for allogeneic hMSC manufacturing – by unit operation
| Stage | Lot Size | Working Cell Bank | Culture Platform | Volume Reduce / Wash | Fill / Finish | Cryopreservation |
| Phase I | 10B | 40M cells in vials / bags | 2D (40 x CS10) | Open or continuous centrifugation | Semi-auto vials, 250/hr | Controlled-rate freezer |
| Phase II | 25B | 100M cells in vials / bags | 3D (50-80 L) | Open or continuous centrifugation | Semi-auto vials, 250/hr | Controlled-rate freezer |
| Phase III / Go-to-market | 100B | 400M cells in bags | 3D (200 L) | Continuous centrifugation | Automated vial line 500+/hr | Controlled-rate freezer |
| Full commercial scale | 500B | 2B cells in bags | 3D (1000 L) | Large scale continuous centrifugation | Fully automated vial line 3000+/hr | Scaled-up controlled-rate freezer |
Proactively Addressing Downstream Processing Bottlenecks
The subsequent post-harvest processing steps involve unit operations that rely on the scale of the expansion platform [2], therefore it is critical to proactively address downstream process unit operations as you choose your expansion technologies. You don’t want to manufacture your products at a scale only to waste a large fraction of it due to incapability of process. Cell harvesting from the bioreactor is immediately followed by a cell-microcarrier separation process. While there are no standard accepted criteria for the number of microcarriers that can be in the final product, near-complete removal of microcarriers from the cell therapy product is critical to ensure safety for the patient. For the microcarrier removal unit operation, screen-based depth filtration technology has been implemented, trapping microcarriers within 60-100 µm meshes while letting cells pass through. This leaves the cells in the quenched harvest reagent for further clarification and concentration.
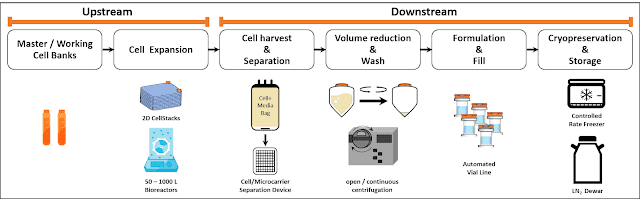
The microcarrier-free cell suspension coming out of the separation step goes into a volume reduction/wash operation. Large scale production of hMSCs (starting at Phase II) demands scalable technologies for this step, i.e. tangential flow filtration (TFF) or a continuous counterflow centrifugation system [2] such as kSep (Sartorius) or Rotea (Scinogy). Newer technologies such as acoustic wave separation technology (e.g. Pall’s Cadence™ Acoustic Separator, FloDesign Sonics) are also being developed and optimized for hMSC manufacturing processes. With a 100B lot size, the kSep is the only platform today that can accommodate 200L volume within a reasonable manufacturing timescale (to give a rough idea, our experience processing 60L of 0.4M cells/ml in the kSep400 with conservative process parameters takes about 1.5 hours in one single cycle, while a typical open centrifugation system for this volume would have taken over 3 hours in 30 consecutive cycles). At 500B cells, the kSep6000S is the only platform that could potentially process the volume associated with this full commercial scale lot size. Other volume reduction technologies such as Rotea or FloDesign Sonics’ Acoustic Wave Technology can be implemented in productions associated with earlier phases or indications associated with smaller lot sizes due to their smaller processing capabilities (10-50B cells).However, keep in mind that these technologies might scale in the future for hMSC manufacturing. Studies can also be done to test these systems for a combined separation/concentration step during the manufacturing steps, but this will require extensive process development and validation for use with cell/microcarrier suspensions.
The envisioned format of the final cell product is a pharma-style vial with ~75M cells/vial at 10 ml fill. To fill 10B cells, a semi-automated vialing system can be used, such as the M1 Filling Station (Aseptic Technologies). For the full-scale production at 500+ vials/hr, a fully automated vialing system such as the Crystal® L1 Robot Line can be employed.
Cryopreservation, followed by storage, is the last step of the manufacturing process. Since the fast, controlled-rate freezing process is critical in maintaining cell health, large-scale cryopreservation platforms such as the CryoMed™ Controlled Rate Freezers (CRF), which allows a custom user-defined recipe, is commonly used. Each process development group will work to optimize a controlled rate freezer recipe that works for their final products, with post-thaw viability and expansion being the performance metric.
After all processes, it is important to verify the final cell product quality. The safety of the cells needs to remain intact, and the critical quality attributes of the cells need to be maintained. RoosterBio performs a general analytic panel to ensure safety and critical quality attributes of hMSCs (including cell performance, identity, and functionality testing), but each final product will have its unique testing requirements and must be performed within the context of each development program. However, our goal is to identify and de-risk the comparability challenges for our customers prior to their scaling, simplifying, and accelerating the process and product development for each intended program.
In conclusion, we have highlighted various existing technology platforms involved in an hMSC manufacturing process. You can think of this exercise as a decision-making tool to help streamline an hMSC clinical manufacturing program. As this tool informs the development team on the unit operation choices associated with each phase, it also helps the team define their requirements and invest in lot-size-appropriate technologies. This leads to a RegenMed company with a clinical pipeline to plan for a multi-year process development program to meet the peak commercial demands for most indications that would demand hMSC treatment.
If you have an interest in discussing scalable production platforms, our complete solutions that radically simplify hMSC process development, or our Process Development Services, please reach out to us at info@roosterbio.com!
References:
- Rowley et al., 2012. Meeting Lot-Size Challenges of Manufacturing Adherent Cells for Therapy. BioProcess International 10(3): 16-22
- Pattasseril et al., 2013. Downstream Technology Landscape for Large-Scale Therapeutic Cell Processing. BioProcess International 11(3): 38-47
- Olsen et al., 2018. Peak MSC—Are We There Yet? Front Med 5:178. doi: 10.3389/fmed.2018.00178 PubMed
- Simaria et al., 2013. Allogeneic cell therapy bioprocess economics and optimization: Single‐use cell expansion technologies. Biotechnol Bioeng 111(1): 69-83. doi: 10.1002/bit.25008 PubMed
