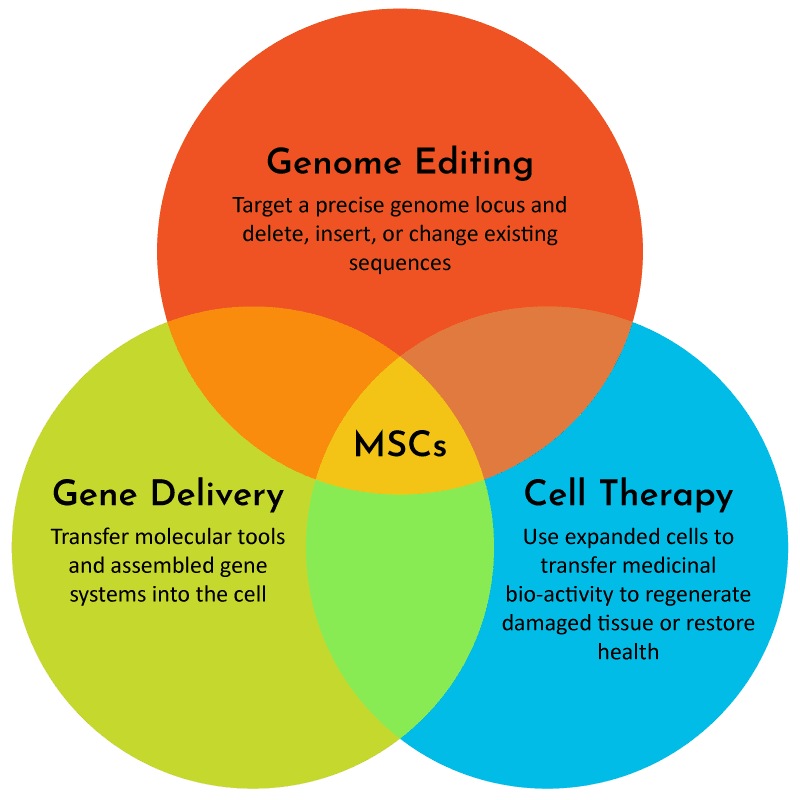
 Above, A Venn diagram showing MSCs at the convergence of major developments for the future of medicine. When MSCs are genome-edited, it will inevitably be done with cell therapy in mind and should involve complementary breakthroughs to scale up and industrialize gene delivery. RoosterBio’s newly published e-Book Genome Editing of MSCs explores this topic.
Above, A Venn diagram showing MSCs at the convergence of major developments for the future of medicine. When MSCs are genome-edited, it will inevitably be done with cell therapy in mind and should involve complementary breakthroughs to scale up and industrialize gene delivery. RoosterBio’s newly published e-Book Genome Editing of MSCs explores this topic.
2020 was a watershed year for genome editing. Drs. Jennifer Doudna and Emmanuelle Charpentier at won a much-anticipated Nobel Prize for their foundational CRISPR work, and their molecular toolkit had entered human patients for the first time. Between FY2011-FY2018, NIH funding for CRISPR-related research, alone, totaled $3.1B, and surely 2020 would substantially add to that tally. Companies dedicated to gene editing (e.g., CLLS, DTIL, CRSP, EDIT, SGMO, NTLA, HZNQF and others) already presently value more than $10 billion in combined market capitalizations, and these promise to revolutionize gene and cell therapies with new cures for diseases and enhanced biofunction to support biologics manufacture.
The present crop of gene-edited cell therapies is grounded in T-cells and HSCs. However, it will be difficult to ignore edited MSCs in the coming decade, since they are workhorses of regenerative medicine. Since the year 2000, the number of MSC-related grants increased more than tenfold, and number of patent application family filings (earliest priority date) with close-matching MSC terminology has increased from ~100 in year 2000 to over 2000 by 2019. PubMed’s roster of related publications yielded similarly prodigious expansion over this same two-decade period. MSCs are regularly featured in over 100 new worldwide interventional clinical trials with each new year (now ~300), and the number of planned enrolled patients per year to enroll has nearly doubled between 2011 and 2019, with 2020’s total of ~20,000 exceeding 2019’s by a large margin. Most importantly, this extensive clinical trial track record has demonstrated that MSCs have an excellent safety profile when administered under carefully monitored conditions. At this stage with 11 commercial MSC-based therapies approved outside of the USA, elusive FDA approvals for the first therapies will almost certainly happen in the 2020s. Will some of these comprise genome-edited MSCs? We believe the answer will be a resounding “yes!”
MSCs were originally considered useful to clinicians due to their extended ex vivo replicative capacity and ability to differentiate into multi-lineage cell fates such as bone, fat, chondrocytes, and possibly several others. To both clinical and commercial entities, however, it’s increasingly now apparent that most utility of MSCs lies not in their multipotency but rather in their ability to secrete factors that mediate tissue repair, modulate innate immunity at a local and systemic level, and perhaps even gravitate to sites of injury or tissue damage. The unique MSC secretome not only includes growth, survival, and protein angiogenic factors, but also mitochondria and exosomes or extracellular vesicles (EVs), which can be leveraged for cGMP bioproduction, analogous to CHO cell protein secretion.
Being amenable to clinical use but lacking the fragility and non-scalability of other primary cells, many envisage MSCs as an ideal plug-and-play “chassis” for the gene programs and systems of clinically applied synthetic biology. Accordingly, recent clinical trials to deploy genetically engineered MSCs as “in situ cellular bioreactors” span several methods of gene transfer and MOAs: HSV-TK-expressing gamma-retrovirus for gastrointestinal cancer (apceth, GmbH, now Minaris), Notch1-expressing plasmid for stroke (SanBio), TRAIL-expressing lentivirus for lung cancer (University College London), IFNb-expressed via plasmid for ovarian cancer (MD Anderson Cancer Center), and oncolytic adenovirus for glioma (MD Anderson Cancer Center).
Gene editing is clearly a step beyond “simple” gene modification in terms of technology. Unlike gene-modified MSCs, gene-edited MSCs have hence not transitioned into IND-enabled human clinical studies. Yet the flight path to a clinic-ready, genome-edited (GE)-MSC ought to be well-navigated within several years. The use of genome editing tools is attractive because edits are a permanent and highly precise manifold to convert an MSC from a mere “cell-drug” into a supercharged drug delivery system, in a fashion far less susceptible to donor variability. To facilitate the transition of MSCs into drug delivery systems, we have prepared an e-Book for your enjoyment in hopes that it will provoke further research and exploration of this new frontier.
If you’re an MSC or cell therapy investigator looking to “kick things up a notch” and want to join the flood of colleagues who re-design the next “MSC 2.0” supercells, look no further than this primer.
This e-Book is well-referenced and has concise charts, tables, and diagrams. It seeks to help provide a perspective on your questions related to:
What is genome editing and what makes this practice distinct from other forms of genetic engineering?
What are the major editing technologies, and the strengths and weakness of each?
How might you best deliver genome editor toolkits temporarily into the nuclei of MSCs for permanent edits?
How do you select, sort, and expand MSCs once you edit a fraction of their population for human cell therapy?
How do you validate whether the MSC end product is edited properly?
How is an MSC like a mobile cellular device?
… and more!
As RoosterBio looks to help facilitate a bright future for the field of MSC-based regenerative medicine with our MSC product system and development services, we invite you to grab a cup of your favorite morning beverage—and then take a few moments to learn how our team sizes up an interesting and growing opportunity that will transform medicine as we know it!

