Analysis performed by and blog authored by Omokhowa Agbojo, Masters of Engineering, BioEngineering, UC Berkeley in collaboration with Maya Lim, PhD and Josephine Lembong, PhD.

In the Fall of 2020, RoosterBio partnered with the University of California, Berkeley Fung Institute’s Master of Engineering program to launch a study into the environmental impacts of human mesenchymal stromal/stem cell (hMSC) manufacturing. The motivation behind this project was not only to understand and quantify the levels of impact of cell therapy manufacturing but also to heighten our awareness of such impacts in years to come. In this project, the environmental impact of hMSC manufacturing has been quantified at two different clinically relevant scales and in three different configurations for production.
Background
The oil & gas, electric utilities, agriculture, and metal manufacturing industries are often cited when discussing those that have significant negative impacts on the environment [1]. However, biotechnology is rarely mentioned in the same category as these industries. The biotechnology industry has grown rapidly since its inception in 1973 [2, 3] and is considered an energy-intensive industry, particularly at large scales [4]. In addition to its energy intensity, the biotechnology industry also consumes notable amounts of water and produces significant amounts of waste. There has been an increased push towards environmental consciousness in the mature biotechnology industry with companies like Biogen, Genentech, and Sanofi committing to reducing the negative environmental impacts of their processes (Figure 1) [5–7]. However, as more novel biotechnologies are created, there is little research being done on the environmental impacts of these technologies.
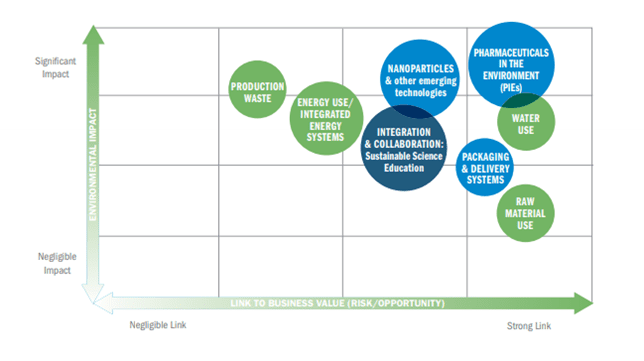 Figure 1: Biogen Materiality Matrix (Biogen workshop in 2015, Cambridge, MA)
Figure 1: Biogen Materiality Matrix (Biogen workshop in 2015, Cambridge, MA)
Large-scale therapeutic cell manufacturing, particularly hMSC manufacturing, is a ready topic for environmental impact, as there are now over 10 hMSC therapeutic products available commercially around the globe and over 40 late-stage clinical trials with multiple more market approvals likely in the next few years.
Today, hMSCs are produced through a sequence of upstream and downstream bioprocessing steps conducted under Good Manufacturing Practice (GMP) guidelines [8] after the initial harvest from a donor. Figure 2 [9] outlines steps in the upstream and downstream production of hMSCs. Within each of these steps, water and energy are consumed and copious amounts of liquid and plastic waste are produced to maintain an acceptable level of sterility in cell culture [10]. Despite the knowledge that hMSC manufacturing produces significant levels of waste and consumes large volumes of water, there is little information about the environmental impacts of the production process.
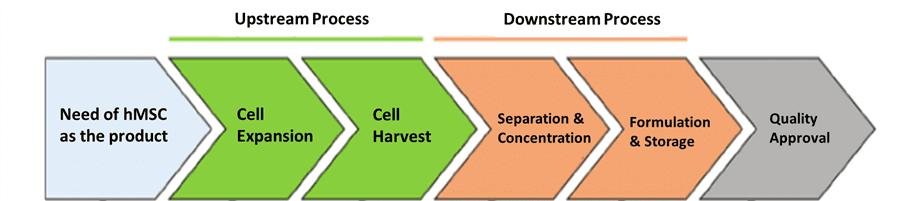
Figure 2: General overview of a dynamic production process for hMSCs (modified for our purposes)
Because of its promise as an impactful therapy for many diseases, hMSC demand has continued to grow significantly [11]. Along with cell therapy, the demand for hMSC-derived products for different applications like cosmetics, synthetic biology, tissue engineering, combination products, clean meat, etc. continues to grow as well. According to Olsen et al., the demand for hMSCs is predicted to increase to multiple sextillions of cells by 2040 [12] – a multi-log increase from today’s levels. As overall demand for hMSCs grows, the scale at which these cells are produced changes from preclinical and laboratory scales to late-stage clinical and even commercial scales [13]. With the increase in demand for hMSCs, the environmental impacts associated with hMSC manufacturing also increase and it becomes even more important to understand and measure the environmental impact to reach the sustainability goals outlined by the rest of the biotech industry.
Clinically Relevant Scales of hMSC Manufacturing
In this project, we analyzed an “early clinical trial scale” which produces approximately 6-10 billion cells. At this scale, hMSC expansion is modeled in two production platforms, 10 layer cell stacks/factories with a growth area of 6,360 cm2 [14] and 15L suspension microcarrier bioreactors, and we compared a traditional media exchange process to a batch production process.
A major component of cell therapy production is cell culture media. Cell culture media is important because it contains all the nutrients that are essential to proper cell growth, and it is often the major cost driver of cell manufacturing bioprocesses. In this study, the environmental impact of the hMSC manufacturing process was evaluated between three unique processes using a portion of the traditional Life Cycle Assessment analysis described below. The three processes used for comparison were:
- “Traditional” cell culture media requiring multiple media exchanges throughout the production process in flasks or 10-layer vessels
- More efficient specialized media designed for a batch (i.e. no media exchange) bioprocess in flasks or 10 layer vessels
- A microcarrier based bioreactor process using an efficient fed-batch culture process with documented enhancements in media productivity [15, 16]
What is Life Cycle Assessment?
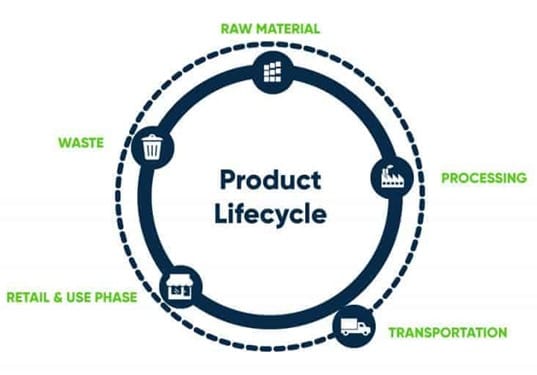
Figure 3: Product Lifecycle Phases, Ecochain.com
Life Cycle Assessment is a method used ubiquitously across many industries to evaluate the environmental impact of a product over its life cycle [17]. It is based on the principle of product life cycles. As seen in Figure 3, a product lifecycle has 5 basic phases: raw material extraction, production and manufacturing, transportation, usage and retail, and waste disposal/end-of-life [18]. For the purpose of this study, we limited our analysis to the PROCESSING stage of the life cycle.
What Did We Learn from Analyzing hMSC Production Processes?
Within the scope of the upstream manufacturing of hMSCs, we evaluated the environmental impacts of switching from a traditional MSC media, which typically requires 2-3x media exchanges between passages, to a highly productive batch bioprocess using commercially available RoosterBio media (RoosterNourish™) that requires no media exchanges. The analysis showed that removing multiple volumes of media exchange in a highly productive bioprocess medium resulted in a vector decrease in all environmental impacts measured (Figure 4). The most significant reductions were found around liquid waste, driven mostly by a reduction in overall media usage, and CO2 emissions that were driven mostly by the increase in handling efficiency between the “traditional” and the RoosterBio processes that reduced the opportunity for CO2 release from incubators. The 12% to 39% decrease in the other factors can be attributed to the elimination of the media change step which contributed a large percentage of the negative environmental impacts.
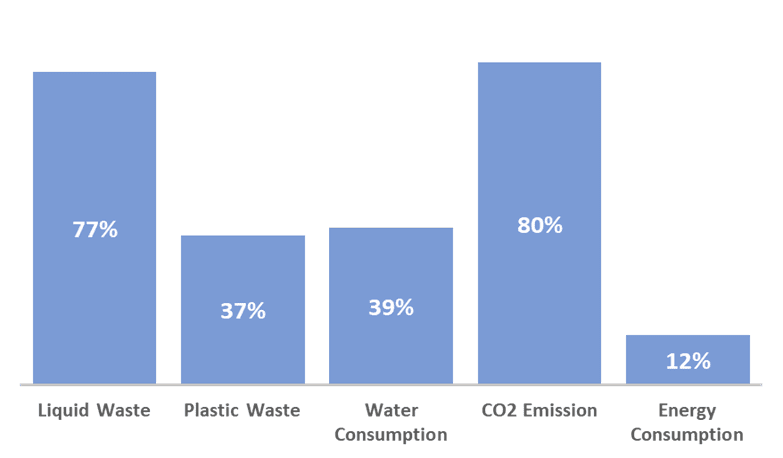
Figure 4: Percent Decrease in Environmental Impact Categories due to a Switch from a Traditional Media Exchange to a Batch Culture process using RoosterNourish Media in CS10s.
In the second part of this study, we delved into the potential benefits and impacts of switching from a flask-based culture system into a scalable fed-batch bioreactor process akin to commercial monoclonal antibody manufacturing. Results of this analysis illustrated an even greater positive impact on the environmental effects of hMSC production when switching from traditional 10-layer flask processes (both “media exchange” and batch) to a fed-batch bioreactor configuration at the clinical scale of 6-10B cells (Figure 5) using RoosterNourish and RoosterReplenish™ products and methods. The largest decreases in environmental impacts observed were in the amounts of plastic and liquid waste produced, as well as in total water consumption. These impacts were significantly influenced by the extensive waste streams associated with traditional culture media and 10-layer manufacturing.
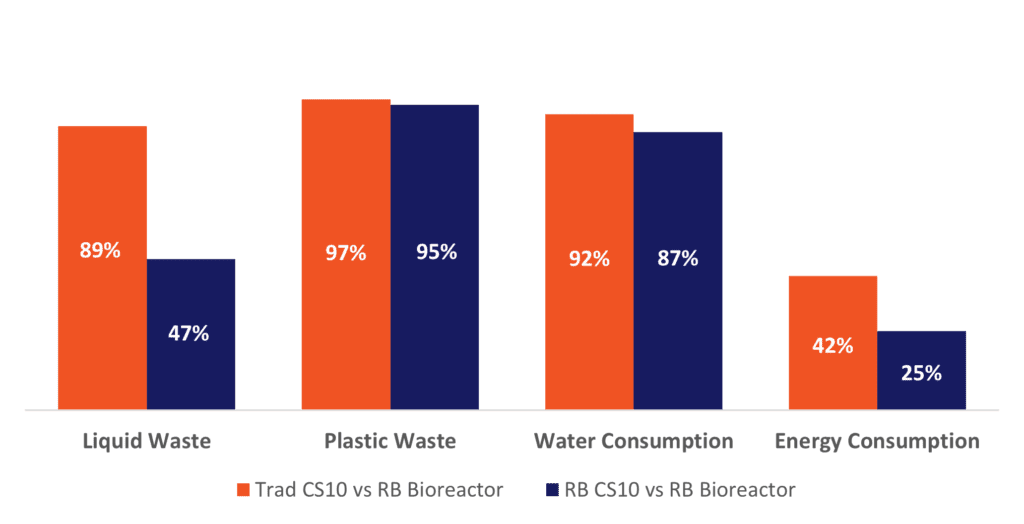
Figure 5: Percent Decrease in Environmental Impact Categories due to the Adoption of a Bioreactor Configuration
Conclusions
The overall outcome from this study truly underscores how technology can not only improve productivity of a manufacturing process but at the same time positively impact our environment. A well-engineered cell culture bioprocess media that drives high productivity in hMSC manufacturing can make a huge impact in the amount of liquid and plastic waste and CO2 emissions as illustrated in this analysis. Further, the transition into scalable platforms such as bioreactors will add greater environmental benefits in terms of plastic waste reduction and overall water consumption.
At RoosterBio, our aim is to solve Regenerative Medicine product and process development challenges with innovative biomanufacturing strategies. We are also driven by social responsibility and committed to making sustainable choices and products. We are excited that this analysis illustrates how RoosterBio manufacturing innovations not only benefit manufacturing cost and scale, but also significantly reduce the environmental impacts of hMSC production. With this analysis in hand, we will continue to innovate with an eye towards sustainable solutions.
References
[1] F. R. S. Inc, “Decomposing Environmental Impacts By Sector.” https://insight.factset.com/decomposing-environmental-impacts (accessed Mar. 15, 2021).
[2] S. N. Cohen, A. C. Y. Chang, H. W. Boyer, and R. B. Helling, “Construction of Biologically Functional Bacterial Plasmids In Vitro,” Proc. Natl. Acad. Sci. U. S. A., vol. 70, no. 11, pp. 3240–3244, Nov. 1973.
[3] J. Smith, “Humble Beginnings: The History of Modern Biotechnology,” Labiotech.eu, Dec. 23, 2020. https://www.labiotech.eu/synbio/history-biotechnology-genentech/ (accessed Mar. 14, 2021).
[4] A. Fairbanks, “Energy Efficiency in Biotech and Pharma.” https://blog.fairbanksenergy.com/energy-efficiency-in-biotech-and-pharma (accessed Mar. 15, 2021).
[5] “2019 Year in Review: Our Commitment to Corporate Responsibility.” https://www.biogen.com/en_us/yearinreview.html (accessed Mar. 14, 2021).
[6] “Genentech: Sustainability Goals & Performance.” https://www.gene.com/good/sustainability/goals-and-performance (accessed Mar. 14, 2021).
[7] “Planet Mobilization.” https://www.sanofi.com/our-responsibility/planet-mobilization (accessed Mar. 14, 2021).
[8] D. E. Rodríguez-Fuentes, L. E. Fernández-Garza, J. A. Samia-Meza, S. A. Barrera-Barrera, A. I. Caplan, and H. A. Barrera-Saldaña, “Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review,” Arch. Med. Res., vol. 52, no. 1, pp. 93–101, Jan. 2021, doi: 10.1016/j.arcmed.2020.08.006.
[9] C. Elseberg, J. Leber, T. Weidner, and P. Czermak, “The Challenge of Human Mesenchymal Stromal Cell Expansion: Current and Prospective Answers,” 2017. doi: 10.5772/66901.
[10] P. Réu, G. Svedberg, L. Hässler, B. Möller, H. A. Svahn, and J. Gantelius, “A 61% lighter cell culture dish to reduce plastic waste,” PLOS ONE, vol. 14, no. 4, p. e0216251, Apr. 2019, doi: 10.1371/journal.pone.0216251.
[11] X. Wei, X. Yang, Z. Han, F. Qu, L. Shao, and Y. Shi, “Mesenchymal stem cells: a new trend for cell therapy,” Acta Pharmacol. Sin., vol. 34, no. 6, pp. 747–754, Jun. 2013, doi: 10.1038/aps.2013.50.
[12] T. R. Olsen, K. S. Ng, L. T. Lock, T. Ahsan, and J. A. Rowley, “Peak MSC—Are We There Yet?,” Front. Med., vol. 5, 2018, doi: 10.3389/fmed.2018.00178.
[13] G. M. Pigeau, E. Csaszar, and A. Dulgar-Tulloch, “Commercial Scale Manufacturing of Allogeneic Cell Therapy,” Front. Med., vol. 5, 2018, doi: 10.3389/fmed.2018.00233.
[14] “Corning® CellSTACK® Culture Chambers | CellSTACK® | Cell Therapy and Vaccines | Browse Products by Application | Life Sciences United States Consumer Site | Corning.” https://ecatalog.corning.com/life-sciences/b2c/US/en/Browse-Products-by-Application/Cell-Therapy-and-Vaccines/CellSTACK%C2%AE/Corning%C2%AE-CellSTACK%C2%AE-Culture-Chambers/p/corningCellSTACKCultureChambers (accessed Mar. 15, 2021).
[15] J. Lembong et al., “Bioreactor Parameters for Microcarrier-Based Human MSC Expansion under Xeno-Free Conditions in a Vertical-Wheel System,” Bioengineering, vol. 7, no. 3, Art. no. 3, Sep. 2020, doi: 10.3390/bioengineering7030073.
[16] J. Lembong et al., “The Need for Adherent Cell Manufacturing: Production Platform and Media Strategies Drive Cell Production Economics,” BioProcess International, Jul. 08, 2020. [Online]. Available: https://bioprocessintl.com/manufacturing/cell-therapies/the-need-for-adherent-cell-manufacturing-production-platform-and-media-strategies-drive-cell-production-economics/
[17] I. V. Muralikrishna and V. Manickam, “Chapter Five – Life Cycle Assessment,” in Environmental Management, I. V. Muralikrishna and V. Manickam, Eds. Butterworth-Heinemann, 2017, pp. 57–75. doi: 10.1016/B978-0-12-811989-1.00005-1.
[18] “Life Cycle Assessment (LCA) – Complete Beginner’s Guide,” Ecochain, May 03, 2019. https://ecochain.com/knowledge/life-cycle-assessment-lca-guide/ (accessed Mar. 15, 2021).
