Overview:
- Scalable downstream processing aims to maximize recovery of hMSC extracellular vesicles from the upstream program while purifying from contaminants.
- Clarification is achieved through successive filtration steps where fouling must be considered.
- Tangential flow filtration (TFF) is a valuable downstream unit operation that can achieve concentration, media exchange, and purification.
As previously presented in an earlier blog article, Big Effects in Small Packages, human mesenchymal stromal cells (hMSCs) produce nano-sized, lipid-bound extracellular vesicles (EVs; a term that includes exosomes) that show immense therapeutic promise in clinical indications. The hMSC extracellular vesicle biomanufacturing program can be described and separated into the upstream, which seeks to maximize hMSC-EV production in culture, the downstream (“DSP”), which facilitates purification and concentration of hMSC-EVs, and the characterization, which ensures extracellular vesicle quality and standardization. Considerations in the upstream process development was covered in a recent blog post: Extracellular Vesicle/Exosome Upstream Process Development.
Achieving large-scale hMSC extracellular vesicle lot sizes to support clinical trials and commercialization requires optimization in upstream process, downstream process, and analytical technologies. From a downstream processing perspective, only a fraction of hMSC-EVs produced upstream will be recovered during downstream purification, and thus maximizing the extracellular vesicle yield and recovery represents the critical goal in process development. This blog post will introduce downstream processing of hMSC-EVs and cover the clarification and tangential flow filtration (TFF) processing steps.
Key Considerations in Extracellular Vesicle Downstream Program Development
Conditioned media produced in the upstream process is a complex mixture that contains not only hMSC extracellular vesicles but also other biological components including cell debris, proteins, nucleic acids, lipids, and media components. Large-scale biomanufacturing of hMSC-EVs for clinical applications will involve a downstream process that concentrates, purifies, and formulates hMSC-EV preparations to meet final product specifications without compromising their critical quality attributes (CQAs).
Conventional methods for laboratory-scale extracellular vesicle concentration and purification include ultracentrifugation (UC), precipitation, and size exclusion chromatography (SEC). For UC, clarified conditioned medium (CCM) experiences centrifugal force ≥100,000x g to pellet MSC-EVs, leaving smaller, lower-density components in the supernatant. Precipitation involves the addition of agents such as polyethylene glycol to sediment extracellular vesicles. SEC provides size-based resolution that removes smaller contaminants from culture.
While product recovery, purity, and formulation efficiency are all important to consider for selection of a suitable downstream process, it is critical to consider scalability in order to meet the ultimate program objectives. These conventional methods present challenges when considering scaleup to larger processing volumes and clinical-grade purity. Scaleup of UC is limited due to equipment constraints, as hundreds of parallel processes would likely be required to handle the hundreds of liters of media produced by a typical large-scale upstream process, which may present process efficiency limitations. Precipitation can lead to co-precipitation of other components, limiting the final sample purity. Since the load volume must typically not exceed 10% of the column volume in SEC processes, scaleup requires a significant amount of SEC resin and extremely large columns which could be cumbersome as a manufacturing process. Scalable options for chromatography purification of hMSC extracellular vesicles will be covered in a future blog article.
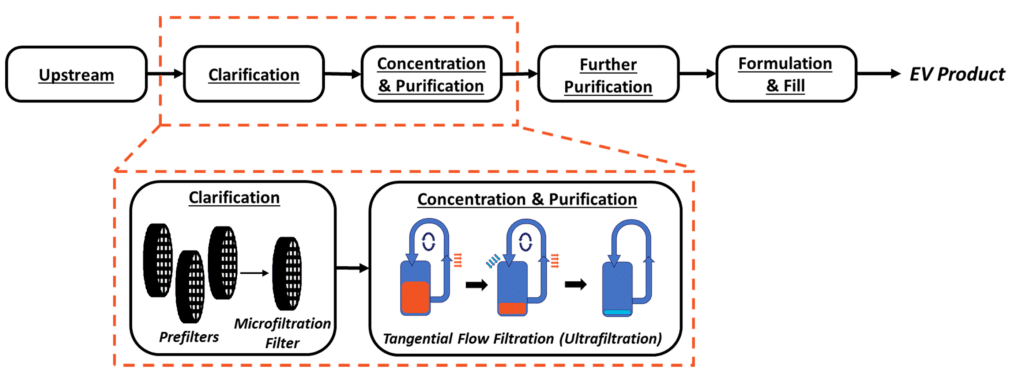
Figure 1. Above. Proposal for a generalized extracellular vesicle downstream process flow. The scope of this blog is indicated by orange
Implementing Multiple Unit Operations in Downstream Processing
A successful downstream program for MSC extracellular vesicles implements a readily scalable, linear process (Figure 1). Since each downstream step feeds directly into the next step, it is important to understand that changes in earlier unit operations will affect each sequential stage. As such, a key consideration throughout downstream development is understanding and optimizing the interfaces between unit operations. CQAs are driving factors in process development decisions and should be evaluated at all stages of development to ensure they are maintained. Some examples of hMSC-EV CQAs routinely monitored at RoosterBio include particle concentration, size distribution, tetraspanin (CD9, CD63, CD81) expression, protein content, and impurity quantification.
Tidying Up Bioreactor Harvest with Clarification
Clarified conditioned medium (CCM) is generated by removing larger debris (>500nm) through a clarification step that terminates in a microfiltration filter. As discussed in Extracellular Vesicle/Exosome Upstream Process Development, 3D bioreactor-based cultures are preferable versus 2D flask-based cultures for scale-up to large volume processes. In bioreactors, the addition of microcarriers (small beads with diameter ~100-500 µm) provides an attachment surface for hMSCs to expand and produce extracellular vesicles.
After harvesting conditioned medium from bioreactors containing hMSC extracellular vesicles, the downstream process must first exclude free microcarriers. Retention rate selection (filter mesh size) is dependent on the size of microcarriers used and the required surface area will be impacted by how conditioned media is harvested from the bioreactor. For example, microcarriers ~125-212 µm diameter will require a filter with mesh size ≤100 µm. Inactivating the bioreactor agitation to allow for microcarrier settling prior to harvest decreases the amount of microcarrier that needs to be removed, and thus also decreases the required filter surface area. Accordingly, harvesting while microcarriers are in suspension will require a significantly larger filter surface area.
After the microcarrier exclusion step, the remaining debris include detached cells, cell debris, and other biologicals and medium components. The key challenge is the selection of an appropriate train of depth and membrane filters of diminishing pore size to minimize the required surface area and thus prevent fouling. This should also provide sufficient recovery and reduction of impurities for an efficient microfiltration step (down to ~0.45 µm). Importantly, the presence of larger debris during microfiltration can lead to significant fouling. To prevent this fouling, coarse and fine depth filters can be used before microfiltration to exclude these larger contaminants without compromising recovery and flow rate through microfiltration filters.
Finally, a terminating microfiltration step should be implemented to remove smaller debris. Depending on the desired outcomes of this unit operation, an absolute 0.2 µm filter may be implemented to prevent microbial contamination. However, this step should be used only when necessary, as it will decrease recovery since many extracellular vesicles are close to, or larger than 0.2 µm.
Checking Three Boxes at Once Through Tangential Flow Filtration
Once CCM is obtained from the filtration step, further processing is necessary to facilitate removal of smaller contaminants while achieving media exchange to substitute bioreactor media components for the formulation media. Tangential Flow Filtration (TFF) is an attractive unit operation that offers product concentration, purification, and medium exchange, and has been successfully implemented as a critical component of downstream processing for hMSC extracellular vesicles by many investigators, including RoosterBio [1-4].
TFF achieves concentration, media exchange, and purification by continuously flowing CCM parallel to an Ultra-Filtration (UF) membrane with molecular weight cutoff (MWCO) 3-6X smaller than the target hMSC extracellular vesicle size [1]. The continuous flow path is achieved by implementing the filter unit into a loop, where the filtrate is expelled out of the path into the permeate, and the remaining media (the ‘retentate’), flows back into the filter unit. Here, smaller impurities are expelled in the permeate, while the great majority of hMSC-EVs are retained. This cycle is repeated until the desired concentration factor is achieved. After concentration, the addition of diafiltration media at a controlled rate leads to a media exchange through continuous diafiltration, which is useful to exchange from the remaining collection medium into a specific buffer composition. Diafiltration is monitored using diavolumes (DVs) (Equation 1), calculated as the ratio of the volume of diafiltration media added to the retentate volume. Increasing DVs increases the degree of medium exchange performed, at the cost of process time.
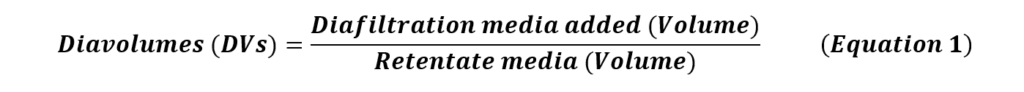
During this step, trans-membrane pressure (TMP), defined as the average of prefilter pressure (Pfeed) and postfilter retentate pressure (Pretentate) subtracted by the permeate pressure (Ppermeate), can be established to improve the permeate flux (Equation 2).
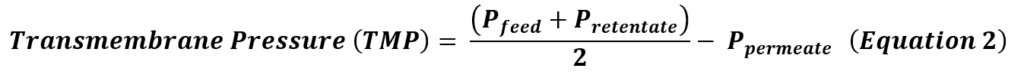
In contrast to normal flow filtration, TFF minimizes filter fouling by virtue of its parallel flow. However, the use of higher TMP during TFF may resemble normal flow and thus may induce fouling, prolonging the processing time. To minimize fouling, the TMP needs to be balanced with an optimized cross flow rate, which is the flow rate across the filter membrane. Crossflow rate is usually defined by the retentate flow rate, which is the flow rate recirculating through the filter module. Given the known geometry of the tubing and membranes, flow rate can be converted to shear rate. High shear rate can affect hMSC extracellular vesicle stability, and thus the flow rate should be optimized. RoosterBio evaluated and optimized the TFF based on the permeate flux, hMSC-EV recovery, and extent of purification, and developed a predictable and scalable process.
For the selection of filter modules, most vendors offer two formats: a set of parallel membrane tubes, known as hollow fibers, or layers of flat membrane sheets, known as cassettes. Generally, hollow fibers are geometrically more efficient and enable a gentler process by utilizing lower shear while cassettes provide higher fluxes and may deliver higher concentration capabilities. Both formats offer advantages, and they should be selected based on key application objectives.
Challenges in TFF arise primarily from the potential nonspecific isolation of impurities based on the MWCO. Impurities such as proteins larger than the MWCO will co-purify with MSC extracellular vesicles. If this occurs, further purification is needed, which can be achieved using further purification steps that will be the subject of a future blog post.
Looking Forward
In this blog post, considerations for the development of a scalable extracellular vesicle downstream process were presented for clarification and TFF unit operations. Following these operations, further purification and formulation are likely required to complete the downstream program. RoosterBio is currently putting these concepts into action to develop a scalable hMSC extracellular vesicle downstream platform to support ongoing clinical trials and to supply the projected hMSC-EV demand. RoosterBio aims to provide the biomanufacturing processes to generate high-quality hMSC-EVs as raw materials to accelerate key development timelines and bring new therapeutics to the market. Subscribe to stay tuned for future blog posts on the development of hMSC extracellular vesicle downstream processing.
References
- Busatto, S., et al., Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells, 2018. 7(12): p. 273.
- Haraszti, R. A., et al., Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol Ther, 2018. 26(12): p. 2838-2847.
- Paterna, A., et al., Isolation of Extracellular Vesicles From Microalgae: A Renewable and Scalable Bioprocess. Front Bioeng Biotechnol, 2022.
- Watson, D. C., et al., Scalable, cGMP-compatible purification of extracellular vesicles carrying bioactive human heterodimeric IL-15/lactadherin complexes. J Extracell Vesicles, 2018. 7(1).
