Scalability of EV Manufacturing is a Major Challenge
Previously, we discussed the emergence of MSC extracellular vesicles (EVs) as clinical therapies. However, a critical barrier in the development of MSC-EVs as a commercial therapy is generating the large amount of EVs that will be required per dose (1). A recent review estimated that the number of exosomes released from 2 million MSCs in 48 hours is equivalent to a single dose for a rodent (2), suggesting that similar to cell therapy, billions of cells will be required to manufacture an EV commercial therapy. In a recent survey, however, the majority of those working with EVs were working with less than 100mL of sample (3) indicating the lack of scalable manufacturing processes in the field. We have identified four keys that we believe are necessary to enable successful manufacturing of EVs for clinical therapies:
- Generating the billions of cells needed with a scalable manufacturing process
- Increasing the number of EVs generated per cell to maximize productivity
- Optimizing downstream EV purification to increase concentration with minimal processing loss (to be addressed in future blog)
- GMP quality supply chain takes years to develop, so starting with the right materials is critical

More Cells Enable Production of More EVs
The first critical challenge to generating the EVs needed for product development and clinical therapies is growing the necessary numbers of cells. RoosterBio (RBI) high-volume cell formats
and paired bioprocess growth medium are engineered for scalable manufacturingto address this bottleneck. Figure 1 illustrates the ability of RBI systems to generate millions to billions of MSCs, which produce trillions of EVs, in an 8 to 10 day process. Our starting Working Cell Bank vial format and culture paradigm decrease manufacturing time and are scalable to yield the number of EVs required for clinical translation.
Scalable Process for MSC-EV Manufacturing
We recently introduced RoosterCollectTM-EV, a low-particle medium that is engineered for EV collection. This medium is designed to be complementary to RoosterBio MSCs and the RoosterBio bioprocess growth medium RoosterNourishTM. Together, these RBI products create a complete system for efficient cell growth and EV collection.
Using these products, the optimized process (shown in Figure 2) is: 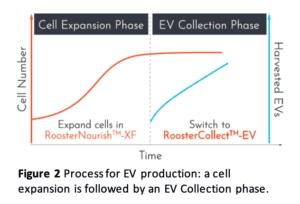
- Expand MSCs in RoosterNourish until at least 80% confluency
- Switch to RoosterCollect-EV
- Collect the conditioned medium
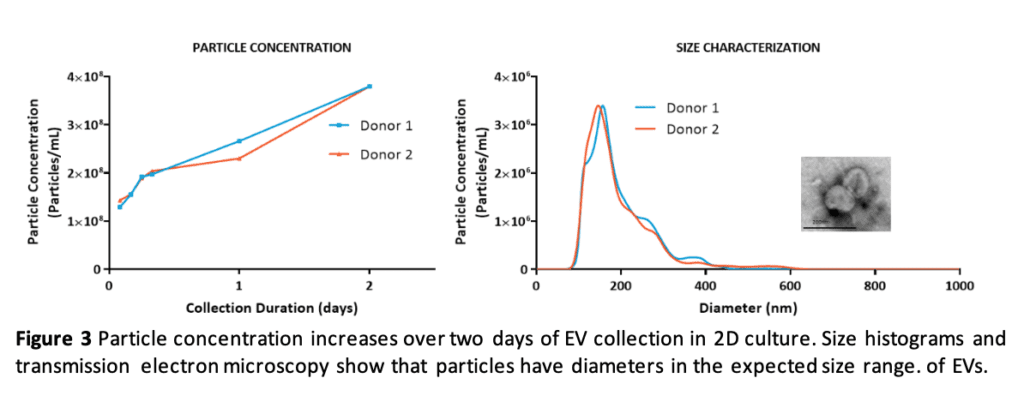
RoosterCollect-EV supports EV collection for at least two days with increasing particle concentration in the conditioned medium and collected particles in the size range expected for EVs (Figure 3).
 This process works for both 2D flask culture and 3D bioreactor culture. Bioreactor culture allows for even greater MSC and EV yields with reductions in cost, labor, and time (see our poster presented at ISCT 2019). Also, conditioned media in 3L and 15L bioreactor culture had greater particle concentration compared to the 2D flask system (Figure 4), which is at least partly explained by the increased cell density we can achieve in bioreactors (see our poster presented at ISEV 2019).
This process works for both 2D flask culture and 3D bioreactor culture. Bioreactor culture allows for even greater MSC and EV yields with reductions in cost, labor, and time (see our poster presented at ISCT 2019). Also, conditioned media in 3L and 15L bioreactor culture had greater particle concentration compared to the 2D flask system (Figure 4), which is at least partly explained by the increased cell density we can achieve in bioreactors (see our poster presented at ISEV 2019). 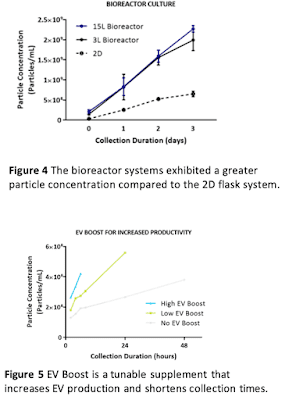
Increased Productivity for More EVs
A complementary strategy to a scalable cell manufacturing process is increasing the EV productivity (i.e. the number of EVs produced per cell). Recently-introduced EV BoostTM is a medium supplement that is designed as a tunable addition to RoosterCollect-EV medium. Depending on the number of EVs required, EV Boost can increase particle yield up to 5x and dramatically shorten collection times (Figure 5).
What’s Next in Scalable EV Production?
Optimizing EV yield will become increasingly important as EVs move to clinical therapies and EVs from billions of cells are required to satisfy dose requirements. RoosterBio’s complete system for MSC-EVs generates millions to billions of cells in 2D or bioreactor culture and trillions of EVs, this system also provides a scalable platform for increasing EV production. This, combined with optimized scalable downstream purification (a third key challenge in EV manufacturing, and subject of a future blog), will enable the success of EVs as clinical therapies.
Rapid translation/transition of to cGMP production will drive the future of scalable EV production for years to come.
References:
- Colao IL, Corteling R, Bracewell D, Wall I. Manufacturing Exosomes: A Promising Therapeutic Platform. Trends Mol Med. 2018;24(3):242-56. https://pubmed.ncbi.nlm.nih.gov/29449149
- Phinney DG, Pittenger MF. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells. 2017;35(4):851-8. https://pubmed.ncbi.nlm.nih.gov/28294454
- Gardiner C, Di Vizio D, Sahoo S, Théry C, Witwer KW, Wauben M, et al. Techniques used for the isolation and characterization of extracellular vesicles: results of a worldwide survey. J Extracell Vesicles. 2016;5:32945. https://pubmed.ncbi.nlm.nih.gov/27802845
