Mesenchymal stem/stromal cells (MSCs) have been clinically translated broadly in the field of regenerative medicine and cellular immunotherapy. These cells possess inherent angiogenic, mitogenic, immunomodulatory, and anti-inflammatory functions, and they can home to injury sites, making them useful for many therapeutic strategies. In addition, the safety profile of MSCs has been demonstrated in over 1,000 clinical trials, including tens of thousands of patients, and they are seen as an ideal “cellular vehicle” for genetic engineering approaches.
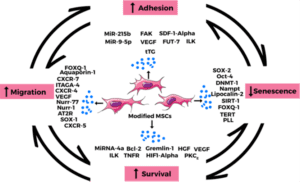
| Figure 1. The focal points of enhancement of MSC properties during modification. MSC modification is geared towards improving their inherent therapeutic properties via enhanced migration, adhesion, survival and reduced senescence. These properties are interdependent and greatly influenced by pretreatment factors and expressed cytokines. (Ocansey et al 2021) |
Genetic engineering of MSCs is aimed at improving their survival and engraftment as well as enhancing their repair mechanisms via modifications aimed at migration, adhesion, and survival of MSCs. (Nowakowski, 2016; McGinley et al, 2011; Ocansey et al, 2020, Figure 1). There are several genetic engineering methods for MSC modification including those implementing viral vectors and those using non-viral techniques, with advantages and disadvantages to each. Of the various approaches, Lentiviral (LV) vectors have been the most widely researched (Figure 2).
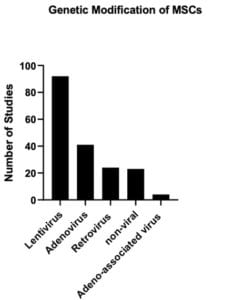
Lentiviral vectors are replication incompetent retrovirus derived from HIV that retain the ability to integrate into the genome of infected cells and have low immunogenicity (Sarkis, 2008). Lentiviruses are therefore beneficial for long-term expression of a therapeutic transgene. Lentiviral vectors have the advantages of stable gene transfer along with high levels of transgene expression (Milone and O’Doherty, 2018) without adversely affecting cell viability, differentiation capacity (McGinley 2011; Lin, 2012), or other MSC Critical Quality Attributes (CQAs) (Dominici, 2006). A general disadvantage associated with the use of integrating viral vectors includes insertional mutagenesis (Apolonia, 2020), which leads to a close regulatory eye over all trials. Low transduction efficiency of primary cells has also been a consistent source of frustration for the advanced therapies field and is a major driver of manufacturing inconsistency in cell-based gene therapy trials.
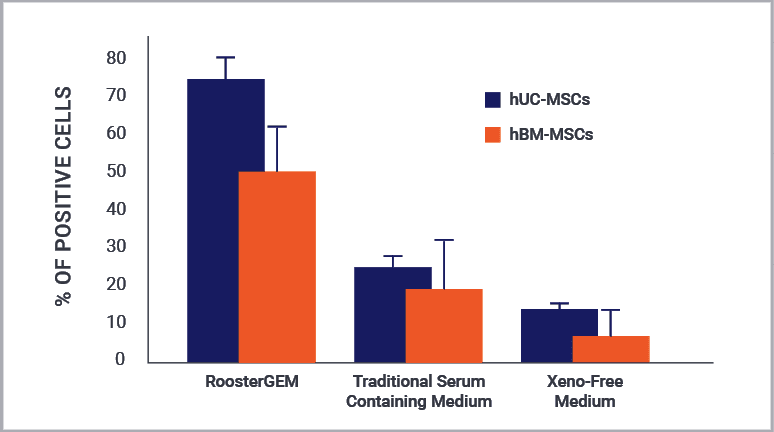
RoosterBio has often been asked to help optimize the lentivirus transduction unit operation of our customers that wished to take a modified MSC into clinical testing. Thus, we developed an optimized genetic engineering medium that we call RoosterGEM™ to support the clinical application of lentiviral transduced MSCs by enhancing transduction efficiencies 2-5 fold over standard expansion media (Figure 3). Using this novel medium, a process to manufacture stably transduced hMSC working cell banks was developed with final product hMSCs having 95% transgene expression, while maintaining typical MSC CQAs (View Poster).
Most recently, lentiviral vectors have been used to transmit microRNA (miRNA) to MSCs to enhance their therapeutic efficacy via MSC-derived extracellular vesicles (EVs). For example, in one study MSC-derived EVs expressing high levels of miRNA were shown to protect against myocardial injury associated with severe acute pancreatitis (Ren, 2021). Another study utilized LV mediated miRNA modification of MSCs as a strategy for the treatment of liver fibrosis (Lou et al, 2017). This work illustrates that, while miRNAs are non-coding RNA sequences, they play important roles in the regulation of gene expression (O’Brien et al, 2018).
Another viral method for engineering MSCs is Adenovirus (AV) vectors which can also infect dividing and non-dividing cells. Due to the low number of AV cell surface receptors on MSCs however, the efficiency of gene delivery is very low. Furthermore, AV have high immunogenicity and transgene expression is transient (Eom, 2020). Adeno-associated virus (AAV) based vectors which exist in an episomal form impart long term transgene expression. However, like AV, AAV have limited clinical application using MSCs because of their low transduction efficiency. Retroviral (RV) vectors also have the disadvantage that they ineffectively transduce MSC and impart transient gene expression. One study however used RV producing MSCs in an animal model of targeted cancer gene therapy (Uchibori et al, 2009). Although non-viral vectors have low immunogenicity and are easy to scale, they suffer from low gene delivery into MSCs. Furthermore, transfection reagents present high toxicity to MSCs. Electroporation and Therapeutic Ultrasound (TUS) have been used reliably in some studies (Eom, 2020). Enhancing the transduction and transfection efficiencies of these other systems for hMSCs is of great interest to the field.
As genetic modification technologies become more “plug and play,” the clinical translation of new therapeutic strategies with MSCs will continue to accelerate. The MSC supply chain is continuing to be industrialized, with off the shelf cell banks for both product and process development, as well as for cGMP manufacturing, already being available. The opportunities for enhancing MSC properties via genetic manipulation are countless, and we foresee an ever-increasing number of clinical trials using engineered MSCs in the very near future.
References
Apolonia L. The Old and the New: Prospects for Non-Integrating Lentiviral Vector Technology. Viruses. 2020; 12:1103.
Damasceno PKF, Alves de Santana T, Santos GC, Orge ID, Silva DN, Albuquerque JF, Golinelli G, Grisendi G, Pinelli M, Ribeiro dos Santos R, Dominici M, Soares MBP. Genetic Engineering as a Strategy to Improve the Therapeutic Efficacy of Mesenchymal Stem/Stromal Cells in Regenerative Medicine. Frontiers in Cell and Devel Biol. 2020; 8:737.
Dominici M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy. 2006;8(4):315-7.
Eom YW, Yoon Y, Sohn JH, Baik SK. Genetic Engineering of Mesenchymal Stem Cells to Improve Therapeutic Effects. J of Stem Cells Research, Development & Therapy. 2020.
Garcia-Bernal D, Garcia-Arranz M, Yanez RM, Hervas-Salcedo R, Cortes A, Fernandez-Garcia M, Hernando-Rodriguez M, Quintana-Bustamante O, Bueren JA, Garcia-Olmo D, Moraleda JM, Segovia JC, Zapata AG. The Current Status of Mesenchymal Stromal Cells: Controversies, Unresolved Issues and Some Promising Solutions to Improve Their Efficacy. Frontiers in Cell and Devel Biol. 2021; 9:650664.
Lin P, Lin Y, Lennon DP, Correa D, Schluchter M,Caplan AI. Efficient Lentiviral Transduction of Human Mesenchymal Stem Cells That Preserves Proliferation and Differentiation Capabilities. Stem Cell Translational Medicine. 2012; 1:886-889.
Lou G, Chen Z, Zheng M, Liu Y. Mesenchymal Stem Cell-Derived Exosomes as a New Therapeutic Strategy for Liver Diseases. 2017; 16:49 (6).
McGinley L, McMahon J, Strappe P, Barry F, Murphy M, O’Toole D, O’Brien T. Lentiviral vector mediated Modification of Mesenchymal Stem Cells & Enhanced survival in an in vitro Model of Ischaemia. Stem Cell Research & Therapy. 2011; 2; 12.
Milone M, O’Doherty U. Clinical Use of Lentiviral Vectors. Leukemia. 2018;32(7):1.
Nowakowski A, Walczak P, Lukomska B, Janowski M. Genetic Engineering of Mesenchymal Stem Cells to Induce their Migration and Survival. Stem Cells Internatioal. 2016; 4956063.
O’Brien J, Hayder H, Zayed Y, Peng C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front Endocrinol. 2018; 9:402.
Ocancey DKW, Pei B, Yan Y, Qian H, Zhang X, Xu W, Mao F. Improved Therapeutics of Modified Mesenchymal Stem Cells: An Update. J Transl Med. 2020; 18:42.
Ren S, Pan L, Yang L, Niu Z, Wang L, Feng H, Yuan M. miR-29a-3p Transferred by Mesenchymal Stem Cell-Derived Extracellular Vesicles Protects Against Myocardial Injury After Severe Acute Pancreatitis. Life Sci. 2021; 1:272:119189.
Sarkis C, Philippe S, Mallet J, Serguera C. Non-Integrating Lentiviral Vectors. Current Gene Therapy. 2008; 8: 430-437.
Uchibori R, Okada T, Ito T Urabe M, Mizukami H, Kume A, Ozawa K. Retroviral Vector-Producing Mesenchymal Stem Cells for Targeted Suicide Cancer Gene Therapy. J Gene Medicine. 2009;11: (5):373-381.
