With acknowledgment to scientists who sounded the alarm like Dr. Liz Specht and to all health workers on the front lines.
What a Successful MSC or MSC-EV Therapy Would Mean for COVID-19 ARDS
Above, Tweet from Liz Specht, PhD, Associate Director of Science & Technology at The Good Food Institute, 6-March-2020. (The Twitter feed discussing calculations behind this tweet).
This influential series of back-of-envelope envelope calculations rang a timely alarm bell for patients in the developed world’s health systems (converted into article form). [1] If the COVID-19 virus does impact within Dr. Specht’s predictions, the USA alone could be facing approximately 2 million cases before the second week in May. By this time, 2 00,000 of these patients would need immediate hospitalization. With 330,000 beds available at any given time, a sizeable proportion of the patients would require stays in the fewer-than-100,000 ICU beds, unable to survive without assistance from 62,000 full featured mechanical ventilators and additional 98,000 emergency breathing devices. [2] This portends a highly concerning duration of shortages of the kind unleashed on Italy, [3] and now in “hot spots” within the USA. While so-called “social distancing” measures might yet provide encouraging evidence for slowing spread, [4] other models spell out possibly dire ramifications to unfold in the coming weeks for the USA, and other sites worldwide. [5] Simply stated, the complex logistics and supply of our 2019 healthcare was not designed to take into account the speed and virulence of 2020’s global respiratory pandemic. Furthermore, today’s 14th-Century methods to fight this virus (“quarantine” comes from Venetian word, quarantena, or 40-days) are accompanied by extraordinary challenges of their own, some without known historical precedent. Thus, COVID-19 urgently demands an innovative technological solution that does not merely “Flatten the Curve,” but one that actually shrinks it.
The World Health Organization reported that recovery from severe COVID-19 can take over a month: [6]
“Using available preliminary data, the median time from onset to clinical recovery is… 3-6 weeks for patients with severe or critical disease. Preliminary data suggests that the time period from onset to the development of severe disease, including hypoxia, is 1 week. Among patients who have died, the time from symptom onset to outcome ranges from 2-8 weeks.”
In addition, Lancet and USA Today report that of the approximately 12% of diagnosed COVID-19 cases needing hospitalization, about 25% of these progress to acute respiratory syndrome (ARDS), requiring intensive care with mechanical respiratory assistance; patients with ARDS are often on respirators for 14 days. [7] [8]
We accordingly plotted (Figure 1) the smoothed average of new USA cases of COVID-19, and conservatively projected that after late-March, the patient doubling time would slow to ~7 days (currently at ~2.5 days in the USA; see Tweet from John Burn-Murdoch, and data available at Github). We then estimate a 32 million-total epidemic to peak in mid-May (~10% of USA), which would taper off (SDev ~30 days, normal distribution) over the next 2 months. Building in a conservative assumption of 3% of diagnosed cases needing hospitalization and 14-day stays in respiratory ICUs, our simple model projects that there will be nationwide critical shortages of fully-featured respirators before 4-May and a 50-day-long oversaturation of each available ICU bed by 8-May. However, if we overlay a shortened time in ICU due to an innovative biotech approach in this model, ICU stay lengths for ARDS could be cut to less than 7 days, and the US medical inventory of ICU beds could reach a shortage by 12-May, experiencing “only” 27 days of oversaturation. Effectively shrinking the entire curve instead of simply flattening it via social distancing.
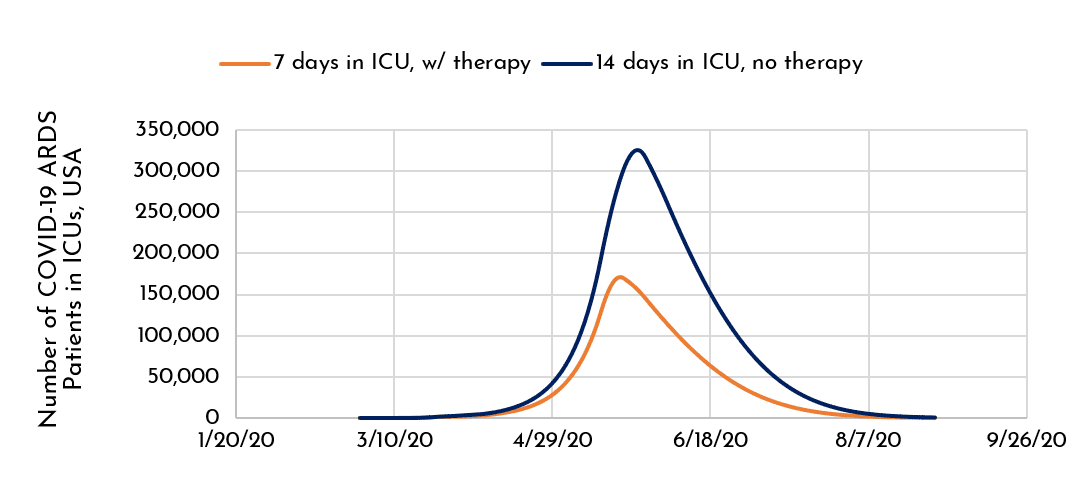 Figure 1. A Modeled COVID-19 Impact on Severely Ill Patients in Respiratory Distress (in USA; -/+ hypothetical therapy).
Figure 1. A Modeled COVID-19 Impact on Severely Ill Patients in Respiratory Distress (in USA; -/+ hypothetical therapy).
What if average critical care time from the ravages of this illness could be shortened 2-fold, from 14 days to 7 days? As shown in the above graph (Figure 1), that potential option could allow more patients to access critical care, saving lives by freeing hospital resources for additional triaged COVD-19 ARDS cases and non-COVID-19 critical patients, alike.
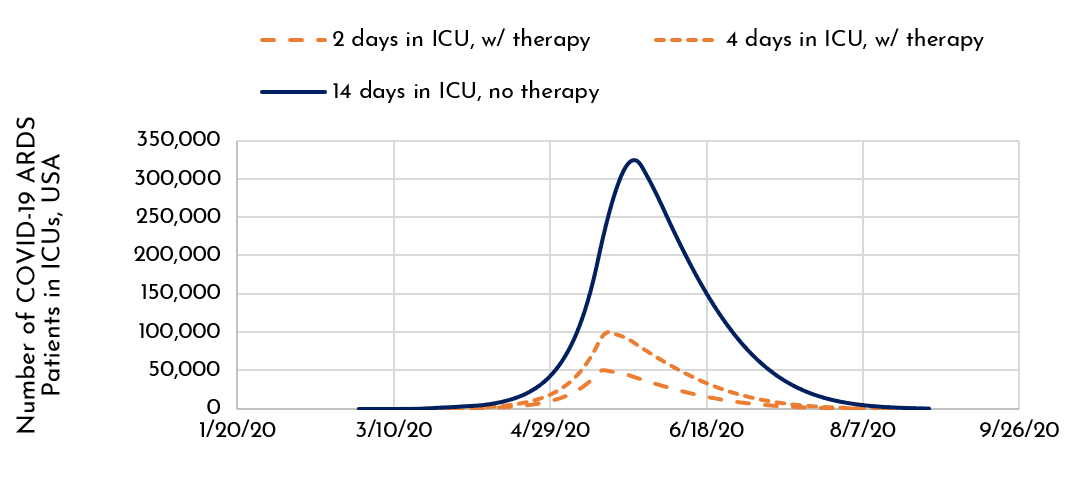 Figure 2. A Modeled COVID-19 Impact on Severely Ill Patients in Respiratory Distress (in USA; -/+ hypothetical therapy that shortens ICU stays to 2 or 4 days).
Figure 2. A Modeled COVID-19 Impact on Severely Ill Patients in Respiratory Distress (in USA; -/+ hypothetical therapy that shortens ICU stays to 2 or 4 days).
Now consider the sanguine prospect that a future therapy could reverse COVID-19 ARDS even more rapidly, allowing patients to exit the ICU after 4 days—or even 2 days. Shown in Figure 2, we compare 14-day ICU stays in which these shorter stays are diagrammed with the orange dotted lines. With 2-4 day stays, we could radically shift the Curve towards the left, and commensurately decrease its weight of impact underneath! …further shrinking the curve of not only today’s COVID-19 pandemic, but of future viral pandemics with an ARDS outcome. In this scenario, for many of these patients and their attending health workers, the supply of ICU beds might be strained but not completely exhausted. It provides the possibility of reducing social distancing and the severe social and economic impact it can have on societies.
As we have blogged earlier, [9] such an alluring possibility is not entirely unreasonable. A recent small study in 7 patients in Beijing YouAn Hospital, China, reported that people with COVID-19 pneumonia could be substantially improved after a treatment with mesenchymal stem/stromal cell (MSC) transplantation. The authors wrote: [10]
“…2-4 days after transplantation, all the symptoms disappeared in all the patients, the oxygen saturations rose to ≥ 95% at rest, without or with oxygen uptake (5 liters per minute). In addition, no acute infusion-related or allergic reactions were observed within two hours after transplantation. Similarly, no delayed hypersensitivity or secondary infections were detected after treatment.”
Furthermore, pre-COVID-19 preclinical work by UCSF’s Dr. Michael Matthay and colleagues via ARDS models of ex vivo perfused human lungs [11] and sheep [12] reported short term physiologic benefits of MSCs (or their secretomes), observable within a matter of hours. As the end-stage of severe COVID-19 infection, the related ARDS can hitherto only be treated with respirators and fluid management.
It’s important to point out that these interesting results are only preliminary, not from optimized materials or protocols, and not intended to invoke claims of actual efficacy. Nevertheless, they do provide a highly compelling rationale for further studies to employ MSCs or MSC secreted products, such as extracellular vesicles/exosomes . An increase in cell culture scale will be necessary to address the MSC-related doses for hundreds of trial subjects and thousands of COVID-19/ARDS patients; these doses must originate from clinical-grade cGMP cells and media with standardized and validated sterility, safety, purity, viability, identity, stability, and potency. [13] Fortunately, as we have reported in a previous blog, the capability to meet this expanded capacity could be within reach, after a relatively modest effort. [14] We have also outlined appropriate manufacturing technology platforms and development paths in our RoosterBio blog.
Based on everything we know, doing our part to “Flatten the Curve” of COVID-19 is sound advice. Yet what would novel and effective treatments do for COVID-19’s impact? The answer is simple: “Shrink the Curve!”
References
- Specht, L. What does the coronavirus mean for the U.S. health care system? Some simple math offers alarming answers. STATnews.com 2020; Available from: https://www.statnews.com/2020/03/10/simple-math-alarming-answers-covid-19/.
- Ventilator Stockpiling and Availability in the US. Johns Hopkins Center for Health Security, centerforhealthsecurity.org 2020; Available from: http://www.centerforhealthsecurity.org/resources/COVID-19/200214-VentilatorAvailability-factsheet.pdf.
- Mounk, Y. The Extraordinary Decisions Facing Italian Doctors. The Atlantic 2020; Available from: https://www.theatlantic.com/ideas/archive/2020/03/who-gets-hospital-bed/607807/.
- Mozingo, J. Why this Nobel laureate predicts a quicker coronavirus recovery: ‘We’re going to be fine’. Los Angeles Times 2020; Available from: https://news.yahoo.com/why-nobel-laureate-predicts-quicker-210318391.html.
- Ferguson, N.M., et al., Impact of non-pharmaceutical interventions (NPIs) to reduce COVID-19 mortality and healthcare demand. London: Imperial College COVID-19 Response Team, March, 2020. 16.
- Organization, W.H., Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). 2020.
- Chen, N., et al., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet, 2020. 395(10223): p. 507-513. 10.1016/S0140-6736(20)30211-7
- Rodriguez, A. Some severe cases of coronavirus could result in brain damage, inability to walk. USA Today 2020; Available from: https://www.usatoday.com/story/news/health/2020/03/19/coronavirus-what-acute-respiratory-distress-syndrome-ards/5066412002/.
- Lim, M. Can MSCs Treat Patients with COVID-19? 2020; Available from: https://www.roosterbio.com/covid-19/can-stem-cells-treat-patients-with-covid-19/.
- Zikuan Leng, R.Z., Wei Hou, Yingmei Feng, Yanlei Yang, Qin Han, Guangliang Shan, Fanyan Meng, Dongshu Du, Shihua Wang, Junfen Fan, Wenjing Wang, Luchan Deng, Hongbo Shi, Hongjun Li, Zhongjie Hu, Fengchun Zhang, Jinming Gao, Hongjian Liu, Xiaoxia Li, Yangyang Zhao, Kan Yin, Xijing He, Zhengchao Gao, Yibin Wang, Bo Yang, Ronghua Jin, Ilia Stambler, Lee Wei Lim, Huanxing Su, Alexey Moskalev, Antonio Cano, Sasanka Chakrabarti, Kyung-Jin Min, Georgina Ellison-Hughes, Calogero Caruso, Kunlin Jin, Robert Chunhua Zhao, Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging and disease: p. 216-228. 10.14336/ad.2020.0228
- Lee, J.W., et al., Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A, 2009. 106(38): p. 16357-62. 10.1073/pnas.0907996106
- Asmussen, S., et al., Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax, 2014. 69(9): p. 819-25. 10.1136/thoraxjnl-2013-204980
- Robb, K.P., et al., Mesenchymal stromal cell therapy: progress in manufacturing and assessments of potency. Cytotherapy, 2019. 21(3): p. 289-306. 10.1016/j.jcyt.2018.10.014
- Jon Carson, I.F., John Getz, and Jon A. Rowley. Scalable MSC Manufacturing Matters in a Rapid Response to COVID-19. 2020; Available from: https://www.roosterbio.com/biomanufacturing/scalable-msc-manufacturing-matters-in-a-rapid-response-to-covid-19/.
