Last week was the 4th Annual IBC Cell Therapy Bioprocessing Conference. IBC (home of the BioProcess International conference) was the first conference organizer to dedicate a focused meeting on Cell Therapy Manufacturing Technologies 4 years ago. Since the first conference in 2011, the growth in the field and conference have been amazing. The attendance has grown from less than 90 in year 1 to over 200 this year. The content has also evolved heavily over the last 4 years, demonstrating a high level of sophistication and maturity in a field that seems “early stage” to those looking in from the outside. The talks this year increased in the amount and quality of data presented. Topics included the impact of automation on the simplification, streamlining, and cost reduction of autologous therapies, the use of Quality by Design (QbD) in bioreactor scale-up and analytical development, advances in tissue engineering and biofabrication techniques, and even 2-year data on marketed products. Phil Vanek, the General Manager of GE Healthcare’s Cell Therapy business, summed it up during his talk where he stated that: GE is interested in 1) big problems, 2) compelling clinical data, and 3) opportunities for “industrialization”, and “Cell Therapy/Regenerative Medicine has all three”.
 |
| Various cell manufacturing and processing devices seen throughout the exhibits at IBC’s 4th Annual Cell Therapy BioProcessing Conference – No BioPrinters (yet!) |
There are many signs that the Cell Therapy field is moving much faster than the protein therapeutics field before it and demonstrating rapid progress. What we have here is a traditional case of “standing on the shoulders of giants”, which has been paraphrased on Wikipedia as “discovering truth by building on previous discoveries”.
Cell Therapy is most definitely standing on the shoulders of traditional biologics manufacturing. Our field is benefitting from many of the technologies that have been developed for the biologics production field, including sterile welding and sealing, single-use disposable bags and bagged reagents, single-use bioreactors, and downstream processing equipment, and process analytical technologies. For today’s cell therapy manufacturing processes, these technologies are ready for implementation, since the lengthy technology development and commercialization stages happened over the last 10 years. This has effectively removed years off development timelines; thus, in many cases, we are seeing the manufacturing process and product development occurs quickly. This “technology acceleration”, based on benefitting from developments in preceding fields, is seen across technology disciplines and has been called the Law of Accelerating Returns by Ray Kurzweil.
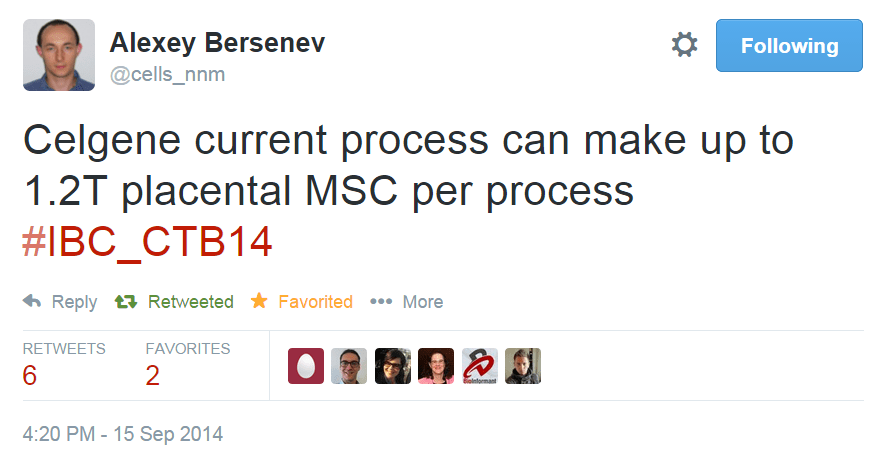 One great example that I have been following of rapid technical progress based on existing technologies is the jump from multilayer vessel manufacturing into suspension bioreactors. In 2005, the field was just beginning to implement hMSC culture in multi-layer vessels – with maximum yield estimates of 5-10 billion cells per harvest. By 2014, we have had two separate big biotech companies announce yields of 300+ billion cells and north of 1 trillion cells in suspension bioreactors using microcarriers. Thus, in less than 10 years, we have seen cell therapy production yields for MSC and MSC-like cells jump over 100 fold – mainly because we were able to benefit from existing microcarrier technologies, existing bioreactor technologies, and quick-to-implement single-use downstream process technologies for cell harvest and concentration. Many of the projections and assumptions that a team from the Process and Product Development Committee of ISCT laid out for lot size limits of adherent therapeutic cells look to have already been met!
One great example that I have been following of rapid technical progress based on existing technologies is the jump from multilayer vessel manufacturing into suspension bioreactors. In 2005, the field was just beginning to implement hMSC culture in multi-layer vessels – with maximum yield estimates of 5-10 billion cells per harvest. By 2014, we have had two separate big biotech companies announce yields of 300+ billion cells and north of 1 trillion cells in suspension bioreactors using microcarriers. Thus, in less than 10 years, we have seen cell therapy production yields for MSC and MSC-like cells jump over 100 fold – mainly because we were able to benefit from existing microcarrier technologies, existing bioreactor technologies, and quick-to-implement single-use downstream process technologies for cell harvest and concentration. Many of the projections and assumptions that a team from the Process and Product Development Committee of ISCT laid out for lot size limits of adherent therapeutic cells look to have already been met!
One last component worth mentioning, which I feel is an under-appreciated aspect of Regenerative Medicine, is biopreservation and cold chain management. The advancements in off-the-shelf and ready-to-use cryopreservation and hypothermic storage solutions are simplifying the logistical challenges of shipping, inventory, and distribution of living cellular products. BioLife Solutions’** CSO Aby Mathews spoke on the impact of biopreservation on logistics and economics of living cellular products. BioLife has had a huge impact on the Cell Therapy field over the last 10 years, and their biopreservation products are now used in over 130 clinical studies and have full Master Files to reference for regulatory filings – making implementation simple and straightforward. While Akron Biotech was not at the conference, they have a new line of cryopreservation products that is being targeted for cell therapy research, and it will be interesting to see how they move towards clinical implementation. Biocision** had Eric Kunkel on the podium to speak about standardization across the cold chain and how their products are designed to fit into the ecosystem. Biocision has been developing a range of devices targeted at Cell Therapy. Their innovative (and cool-looking) products include “ice-free” sample cooling and freezing devices, their CoolCell freezing containers, and validatable inventory transfer chests. Additionally, their newest product, the ThawStar, is focused on the “last mile” of product use, i.e. the controlled thawing of a cell product at the clinical site. It is these types of biopreservation and cold chain products that are making Cell Therapy products better, simpler to implement, and thus, will accelerate the time to market. The field still has the regulatory framework to navigate, but overall the path is much clearer today than it ever has been.
**Full disclosure: Jon Rowley, author of this post, is on the Scientific Advisory Boards of both BioLife and Biocision but holds no stock in either company and was not compensated for this post.