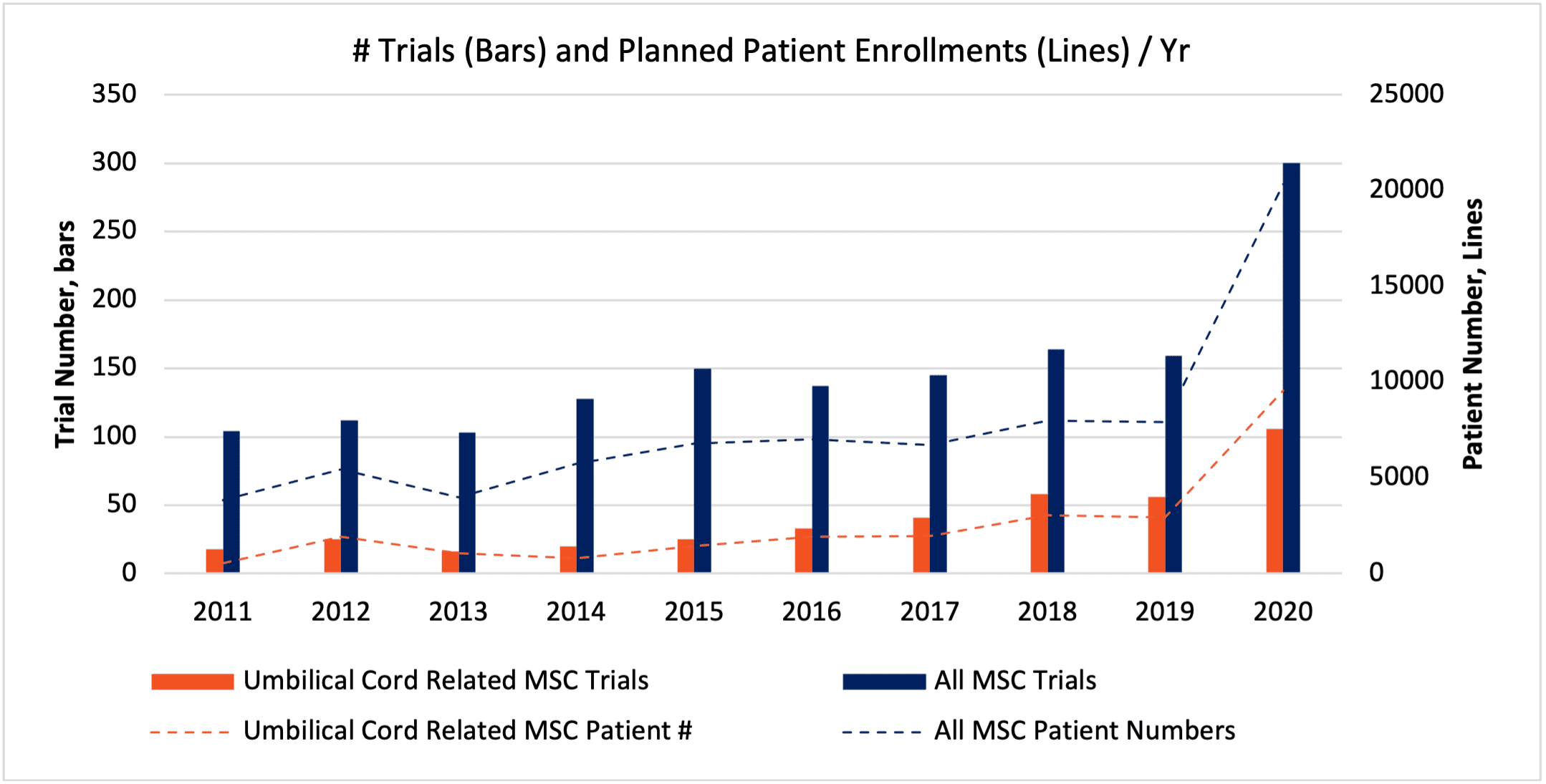
Above, Fig. 1, the number and fraction of MSC trials and patients related to cells obtained via umbilical cord tissues has increased substantially over the last 10 years. (SOURCE: celltrials.org).[1]
After several decades of preclinical research, over 1000 clinical trials, and tens of thousands of patients, mesenchymal stromal/stem cells (MSCs) have exhibited an excellent safety record, as trial growth continued undiminished through the year 2020. Of these cells, the growth in interest for clinical uses of MSCs with umbilical cord origin (UC-MSCs) has been the most prolific of all. Yet, as the field of regenerative medicine advances, we increasingly learn that there are notable differences between MSCs that are sourced from various individual donors, isolation methods, and specific tissues and/or tissue layers.[2, 3] In this blog, we summarize the contrasting features of UC-MSCs vs. MSCs from other tissues of origin, and we suggest where lingering questions could remain.
The International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell (ISCT MSC) Committee establishes broad criteria to define MSCs by morphology, plastic-adherent growth, canonical surface marker expression (-/+), and the stimulated trilineage differentiation capability into osteoblasts, adipocytes, and chondrocytes in vitro. [4, 5, 6, 7] ISCT and MSC advocates alike justifiably caution against the use of the term “stem cell” to describe MSCs, since MSCs do not routinely differentiate into more than a small set of cell types, and do not exhibit perpetual stem-like self-renewal.[8] Nevertheless, when isolated and propagated under carefully prescribed media and conditions, UC-MSCs do demonstrate qualities that are very favorable as the “chassis” for many cell engineering toolkits.[9, 10, 11, 12, 13, 14]
|
MSC Type |
Tissue Collection | MSC Isolation Ease | Propagation / Expansion |
Comment |
| BM-MSC (Bone Marrow) | Very challenging | High success | Intermediate (donor dependent) | Longest exposure to patients across the history of clinical trials. Lower tissue factor (TF) expression, consistent trilineage differentiation and immunosuppressive properties. |
| AT-MSC (Adipose) | Highest volume | High success | Intermediate (donor dependent) | Higher proclivity to show adipogenesis on stimulation. Higher TF expression. Appealing for autologous therapies due to high volumes of fresh cells available at bedside procedures. |
| UC-MSC (Umbilical Cord) | Simple collection & established banking systems | Challenging and/or proprietary methods to meet ISCT guidelines for MSC identity. | Most prolific expansion | Less consistent trilineage differentiation, depending on source & methods. Higher TF expression. Yet lower cell age and MHC -/- makes them attractive for allogeneic cell tx. developers. |
Above (Table 1), salient features of three major types of MSCs used in regenerative medicine clinical trials.
Let’s first highlight what make UC-MSCs most uniquely appealing. These MSCs can grow(!),[15] both in terms of reduced population doubling time as well as in dramatically increased numbers of potential cell doubling cycles, compared with other MSC types.[16, 17] Consistent with their perinatal origin, they express the lowest level of molecular markers of senescence of the MSCs.[18] This feature can allow them to be more readily manipulated “upstream” in the cell manufacturing process, such as through a hypoxic[19] or cytokine[20] preconditioning, sorting or selections, or genetic modifications and/or edits.[14, 21, 22] That is, extra expansion time frees up our options to pursue upstream preparation methods. These can be instrumental for cellular product uniformity, and in turn, less variable performance in a dosed patient population. Also, some specialized preparation methods of UC-MSC (e.g., via perivascular cells of Wharton’s Jelly tissue)[23] yield cells that show uniquely low or non-existent surface expression of MHC complexes,[16] in addition to a strong immunosuppressive paracrine phenotype. These would thus plausibly be hypothesized to demonstrate longer in vivo persistence, and superior homing. Nevertheless, one in vivo study finds that UC-MSCs transplanted intramuscularly can exhibit superior anti-inflammatory properties vs. adult MSCs on the contralateral site of experimental inflammation; this systemic effect is not dependent on MSC homing, but rather on amplified cross-talk with the innate immune system.[24] In “accord” with these and other related observations, the worldwide scope of UC-MSC trial activity is almost universally aimed at allogeneic administration, not autologous.[1, 25]
MSC supernatants in collection media are a known source of raw material for extracellular vesicles (EVs) and/or exosomes.[26, 27, 28] EVs are under clinical investigation as therapeutic raw materials, either alone or as custom-modified particles for controlled drug delivery.[29, 30, 31] Although this topic remains fertile ground for fascinating new studies,[27] extracellular vesicles/exosomes from UC-MSCs already appear to demonstrate properties that are tissue origin-specific.[32, 33] One such study analyzed the proteomics of 1014 unique EV proteins from BM-, AT-, and UC-MSCs, and found similar and differential properties amongst these.[34] While bone marrow MSC-EV proteomes seemed to cluster more towards immune signal transduction—and AT-MSC-EVs were more related to the nervous system or phospholipids—the UC-MSC-EVs appeared most enriched with proteins involved with vascular repair. As expected, exosome-EVs from all three MSC types shared expression of the tetraspanins and extracellular matrix (ECM)-receptor related proteins. Incidentally, UC-MSCs would also appear to be (by far) most prolific among the major subtypes for per-cell EV productivity, according to studies by Dr. Anastasia Kvorova’s group at UMass.-Worcester.[35] Further, their productivity is enhanced further when these UC-MSCs are grown in 3D bioreactors on microcarriers. We have ourselves validated excellent growth of UC-MSCs on microcarriers[36] in a model bioreactor system, and shown in different hMSCs that 3D microcarrier growth of MSCs supports increased EV production vs. growth in 2D flasks.[26] High UC-MSC productivity in scalable bioreactor systems translates larger dose numbers for patients in need of EV therapeutics.
Do UC-MSCs supply a universal or niche opportunity within the larger group of MSC-based therapies? Comparing UC-MSCs with two other major common tissue sources [9, 37] (see Table 1), the tissue collection and cryo-banking would seem to be a simple matter. Up to 380,000 healthy babies are born each day, and thousands of cords are banked, especially in nations that support high rates of practitioners (e.g., Singapore, ~30%).[38] Cells from afterbirth tissues are from a less-stressed source, having undergone fewer divisions since conception, and are protected from toxic insults by the physiologic and developmental choreography that nurtured our species’ survival across 200,000 years of evolution. Though cosmetic adipectomies supply a high volume of AT-MSCs per procedure, those adipose MSCs are from an adult (and possibly aged) source, and the procedure is invasive; so, different individual lifestyle choices could profoundly affect the product quality. Bone marrow aspirate donations for BM-MSCs are painful, and these also can show varied phenotypes based on one’s health, age, and environmental exposures. The post-collection processing to obtain nearly population-identical UC-MSCs is understood to be more of a challenge than AT-MSCs or BM-MSCs, however—and their track record in the clinic is not as long as BM-MSCs’. For therapeutic applications, some experience suggests that BM-MSC might be better suited for chondrogenic indications,[39] while AT-MSCs are been proposed to demonstrate more robust differentiation plasticity for a broad range of in situ injected disease lesions.[40] Yet, if some experimental [41, 42] and omics [9, 43] data are reproducible, it’s possible that UC-MSCs demonstrate a stronger potential to differentiate into hepatocyte-like cells. Generalizable comparisons across MSC types can, of course, be challenging due to context-dependent experimental conditions and donor-to-donor variability within MSC types.[3, 44, 45]
Stating the obvious (to most of us?), all UC-MSCs might not add up to the panacea to support all cellular medicines. The umbilical cord is a complex, heterogeneous tissue with varied layers, compartments, and cell types. Although cord blood is a widely used source for UB-MSCs, the portion of the cord that is most enriched with MSCs appears to be the Wharton’s Jelly (WJ). Fractions of WJ (e.g., perivascular zone)[46] must be surgically and/or enzymatically isolated to be free of blood cells, endothelial cells, and epithelial cells.[37] The requirement for specialized expertise to obtain pure populations of higher-performance UC-MSCs by way of the WJ is high, and pursuant to ongoing technology innovation. [21, 47, 48, 49] Thus, despite recent exuberance in the trial activity (see Figure 1 in this document) also reflecting enduring clinical interest,[25, 50] it’s probably safe to assume that “not all UC-MSCs are created equal.”[46, 51] Some reports in the research literature do suggest that UC-MSCs might be challenging to demonstrate full, in vitro trilineage differentiation into adipocytes, chondrocytes, and osteoblasts;[52] taken at face value, could this imply that a few so-called UC-MSC products would skirt the essential criteria of how the ISCT defines as an “MSC?” Would some be more precisely defined as “fibroblasts”—others, a more “primitive” cell type that propagates with greater inertia along a less differentiated path? Cellular product developers, take note: when you opt to use UC-MSCs in your preclinical and clinical development, be sure to request documentation to show that your umbilical cord donor preparation has all the phenotypic markers that are important. And do “test drive” the cells and donor sources such that they repeatedly perform up to high standards, in your own lab’s “hands.”[53]
To summarize, UC-MSCs may be outstanding for many allogeneic, off-the-shelf uses for industrial production scales, whether for extracellular vesicle biomanufacturing or for cells that are directly injected (see Table 1). On a final note, a few reports describe that UC-MSCs and AT-MSCs can express higher amounts of biofunctional tissue factor (TF) than BM-MSCs, which correlates with reduced clotting times via in vitro assays.[44, 54] Although direct clinical cautions are so far unknown, perhaps it’s worth positioning the use of IV-injected UC-MSCs away from diseases where coagulopathy is a factor—instead preferentially aiming these MSC products for localized, intramuscular, or encapsulated sites of injection? Alternatively, TF might prove to be a worthwhile target for artificial silencing or INDEL genome editing in future UC-MSC clinical products.
As we look to the bright future of UC-MSCs, some key questions remain to be answered. Among them are:
How can we best industrialize sourcing, isolation, and propagation of UC-MSC for high-quality, standardized cellular therapy products?
How can we approach experimentation to reconcile some differences seen between functional cellular phenotypes observed in vitro and in vivo, or even between animal models and human patients?
Are some routes of administration for UC-MSC doses more efficacious than others, and for which clinical indications?
What is the mechanism behind the signal of physiologic (and likely therapeutic) effects seen via UC-MSC and/or UC-MSC-EVs? Are these mediated in a direct paracrine manner, or are they amplified through communication with other cell types?
Given the massive scaleup in production infrastructure for clinical grade mRNA (e.g., Pfizer-BioNTech’s and Moderna’s COVID-19 immunizations), can this industrialized supply chain help remove barriers to entry for “spin-off” innovations & breakthroughs for new therapies related to mRNA-transfected UC-MSCs?
Though these and other questions remain, one thing is certain. The voice of the regenerative medicine community has spoken, and its demand to clinically evaluate UC-MSCs and their secreted products is high. Perhaps it’s most appropriate to ask not what UC-MSCs can do for you, but rather what you can do *to* your UC-MSCs… for regenerative medicine’s future treatments and cures.
RoosterBio stands ready to meet these needs with its ongoing rollout of new MSC, media, and high-efficiency bioproduction formats to support your clinical development and scale-up. Stay tuned! There’s much more on the way.
References
- celltrials.org. Cell Trials Data. celltrials.org 2021; Available from: https://celltrials.org/cells-data
- Ujiie, Yuko, Kazuhiro Gomi, and John Edward Davies, MSC functional phenotype: Assay, age and source dependence. Science Proceedings, 2015. 2.
- Hass, R., et al., Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal, 2011. 9: p. 12. 10.1186/1478-811X-9-12
- Horwitz, E. M., et al., Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy, 2005. 7(5): p. 393-5. 10.1080/14653240500319234
- Viswanathan, S., et al., Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy, 2019. 21(10): p. 1019-1024. 10.1016/j.jcyt.2019.08.002
- Rowley, J.; Farrance, I, Carson, J. What Are MSCs? 2020; Available from: https://www.roosterbio.com/blog/what-are-mscs/.
- Dominici, M., et al., Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy, 2006. 8(4): p. 315-7. 10.1080/14653240600855905
- Caplan, A. I., Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med, 2017. 6(6): p. 1445-1451. 10.1002/sctm.17-0051
- El Omar, R., et al., Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies? Tissue Eng Part B Rev, 2014. 20(5): p. 523-44. 10.1089/ten.TEB.2013.0664
- Zhao, F., et al., Umbilical cord blood mesenchymal stem cells co-modified by TERT and BDNF: a novel neuroprotective therapy for neonatal hypoxic-ischemic brain damage. Int J Dev Neurosci, 2014. 38: p. 147-54. 10.1016/j.ijdevneu.2014.06.014
- Chen, G., et al., Human umbilical cord-derived mesenchymal stem cells do not undergo malignant transformation during long-term culturing in serum-free medium. PLoS One, 2014. 9(6): p. e98565. 10.1371/journal.pone.0098565
- Hei, W. H., et al., Adenovirus vector-mediated ex vivo gene transfer of brain-derived neurotrophic factor (BDNF) tohuman umbilical cord blood-derived mesenchymal stem cells (UCB-MSCs) promotescrush-injured rat sciatic nerve regeneration. Neurosci Lett, 2017. 643: p. 111-120. 10.1016/j.neulet.2017.02.030
- Lee, J., et al., CRISPR/Cas9 Edited sRAGE-MSCs Protect Neuronal Death in Parkinsons Disease Model. Int J Stem Cells, 2019. 12(1): p. 114-124. 10.15283/ijsc18110
- Willstaedt, TM, et al., Development of an optimized lentiviral transduction medium and process to manufacture genetically modified MSC working cell banks. Cytotherapy, 2021. 23(5): p. S45.
- Farrance, Iain. Meeting the growing needs of the perinatal RegenMed Industry: the only Umbilical Cord hMSC (hUC-MSC) system designed for today’s translationally focused research and product development. RoosterBio Blog 2019; Available from: https://www.roosterbio.com/mscs-characterization/meeting-the-growing-needs-of-the-perinatal-regenmed-industry-the-only-umbilical-cord-hmsc-huc-msc-system-designed-for-todays-translationally-focused-research-and-product-development/.
- Sarugaser, R., et al., Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells, 2005. 23(2): p. 220-9. 10.1634/stemcells.2004-0166
- Kern, S., et al., Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells, 2006. 24(5): p. 1294-301. 10.1634/stemcells.2005-0342
- Jin, H. J., et al., Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int J Mol Sci, 2013. 14(9): p. 17986-8001. 10.3390/ijms140917986
- Tsang, W. P., et al., CD146+ human umbilical cord perivascular cells maintain stemness under hypoxia and as a cell source for skeletal regeneration. PLoS One, 2013. 8(10): p. e76153. 10.1371/journal.pone.0076153
- Hamidian Jahromi, S., Y. Li, and J. E. Davies, Effect of Tumor Necrosis Factor Alpha Dose and Exposure Time on Tumor Necrosis Factor-Induced Gene-6 Activation by Neonatal and Adult Mesenchymal Stromal Cells. Stem Cells Dev, 2018. 27(1): p. 44-54. 10.1089/scd.2017.0179
- Sivalingam, J., et al., Biosafety assessment of site-directed transgene integration in human umbilical cord-lining cells. Mol Ther, 2010. 18(7): p. 1346-56. 10.1038/mt.2010.61
- Willstaedt, T. Genetically Engineered Mesenchymal Stromal Cells: A Promising Tool for the Successful Delivery of Therapeutic Genes. RoosterBio Blog 2021; Available from: https://bit.ly/3jp6ybY.
- Ennis, J., et al., Isolation, characterization, and differentiation of human umbilical cord perivascular cells (HUCPVCs). Methods Cell Biol, 2008. 86: p. 121-36. 10.1016/S0091-679X(08)00007-1
- Hamidian Jahromi, S., et al., Human Umbilical Cord Perivascular Cells and Human Bone Marrow Mesenchymal Stromal Cells Transplanted Intramuscularly Respond to a Distant Source of Inflammation. Stem Cells Dev, 2018. 27(6): p. 415-429. 10.1089/scd.2017.0248
- Bartolucci, J., et al., Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res, 2017. 121(10): p. 1192-1204. 10.1161/CIRCRESAHA.117.310712
- Adlerz, K., et al., Scalable manufacturing system for MSC-EV generation. Cytotherapy, 2020. 22(5, Supplement): p. S46. https://doi.org/10.1016/j.jcyt.2020.03.051
- Alvarez-Viejo, M., Mesenchymal stem cells from different sources and their derived exosomes: A pre-clinical perspective. World J Stem Cells, 2020. 12(2): p. 100-109. 10.4252/wjsc.v12.i2.100
- Adlerz, K., et al., Strategies for scalable manufacturing and translation of MSC-derived extracellular vesicles. Stem Cell Res, 2020. 48: p. 101978. 10.1016/j.scr.2020.101978
- Kalluri, R. and V. S. LeBleu, The biology, function, and biomedical applications of exosomes. Science, 2020. 367(6478). 10.1126/science.aau6977
- Haraszti, R. A., et al., Serum Deprivation of Mesenchymal Stem Cells Improves Exosome Activity and Alters Lipid and Protein Composition. iScience, 2019. 16: p. 230-241. 10.1016/j.isci.2019.05.029
- Carson, Jonathan. It’s Not Rocket Science – “MSC-EVs” Have the Right Stuff. 2021; Available from: https://bit.ly/3B7Byn8.
- Hoang, D. H., et al., Differential Wound Healing Capacity of Mesenchymal Stem Cell-Derived Exosomes Originated From Bone Marrow, Adipose Tissue and Umbilical Cord Under Serum- and Xeno-Free Condition. Front Mol Biosci, 2020. 7: p. 119. 10.3389/fmolb.2020.00119
- Bakhtyar, N., et al., Exosomes from acellular Wharton’s jelly of the human umbilical cord promotes skin wound healing. Stem Cell Res Ther, 2018. 9(1): p. 193. 10.1186/s13287-018-0921-2
- Wang, Z. G., et al., Comprehensive proteomic analysis of exosomes derived from human bone marrow, adipose tissue, and umbilical cord mesenchymal stem cells. Stem Cell Res Ther, 2020. 11(1): p. 511. 10.1186/s13287-020-02032-8
- Haraszti, R. A., et al., Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol Ther, 2018. 26(12): p. 2838-2847. 10.1016/j.ymthe.2018.09.015
- Takacs, Joseph and Adlerz, Katrina. hUC-MSC Exhibit Robust Proliferation in 3D Bioreactor System. 2020; Available from: https://www.roosterbio.com/cell-therapy/huc-msc-exhibit-robust-proliferation-in-3d-bioreactor-system/.
- Garcia-Munoz, E. and J. Vives, Towards the standardization of methods of tissue processing for the isolation of mesenchymal stromal cells for clinical use. Cytotechnology, 2021: p. 1-10. 10.1007/s10616-021-00474-3
- Verter, Frances PhD. Percentage of births banking cord blood by country. 2020; Available from: https://parentsguidecordblood.org/en/news/percentage-births-banking-cord-blood-country.
- Contentin, R., et al., Comparison of the Chondrogenic Potential of Mesenchymal Stem Cells Derived from Bone Marrow and Umbilical Cord Blood Intended for Cartilage Tissue Engineering. Stem Cell Rev Rep, 2020. 16(1): p. 126-143. 10.1007/s12015-019-09914-2
- Mazini, L., et al., Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int J Mol Sci, 2019. 20(10). 10.3390/ijms20102523
- Anzalone, R., et al., New emerging potentials for human Wharton’s jelly mesenchymal stem cells: immunological features and hepatocyte-like differentiative capacity. Stem Cells Dev, 2010. 19(4): p. 423-38. 10.1089/scd.2009.0299
- Yu, Y. B., et al., Differentiation of umbilical cord mesenchymal stem cells into hepatocytes in comparison with bone marrow mesenchymal stem cells. Mol Med Rep, 2018. 18(2): p. 2009-2016. 10.3892/mmr.2018.9181
- De Kock, J., et al., Mesoderm-derived stem cells: the link between the transcriptome and their differentiation potential. Stem Cells Dev, 2012. 21(18): p. 3309-23. 10.1089/scd.2011.0723
- Gregoire, C., et al., Comparison of Mesenchymal Stromal Cells From Different Origins for the Treatment of Graft-vs.-Host-Disease in a Humanized Mouse Model. Front Immunol, 2019. 10: p. 619. 10.3389/fimmu.2019.00619
- Li, J., et al., Comparison of the biological characteristics of human mesenchymal stem cells derived from exfoliated deciduous teeth, bone marrow, gingival tissue, and umbilical cord. Mol Med Rep, 2018. 18(6): p. 4969-4977. 10.3892/mmr.2018.9501
- Davies, J. E., J. T. Walker, and A. Keating, Concise Review: Wharton’s Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells. Stem Cells Transl Med, 2017. 6(7): p. 1620-1630. 10.1002/sctm.16-0492
- Shohara, R., et al., Mesenchymal stromal cells of human umbilical cord Wharton’s jelly accelerate wound healing by paracrine mechanisms. Cytotherapy, 2012. 14(10): p. 1171-81. 10.3109/14653249.2012.706705
- Kim, D. W., et al., Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci, 2013. 14(6): p. 11692-712. 10.3390/ijms140611692
- Kita, K., et al., Isolation and characterization of mesenchymal stem cells from the sub-amniotic human umbilical cord lining membrane. Stem Cells Dev, 2010. 19(4): p. 491-502. 10.1089/scd.2009.0192
- Borow, K. M., et al., Phase 3 DREAM-HF Trial of Mesenchymal Precursor Cells in Chronic Heart Failure. Circ Res, 2019. 125(3): p. 265-281. 10.1161/CIRCRESAHA.119.314951
- Cozene, B. M., et al., Mitochondrial activity of human umbilical cord mesenchymal stem cells. Brain Circ, 2021. 7(1): p. 33-36. 10.4103/bc.bc_15_21
- Rebelatto, C. K., et al., Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood), 2008. 233(7): p. 901-13. 10.3181/0712-RM-356
- XF hUC-MSC Donor Screening Kit. 2021; Available from: https://bit.ly/2TdFmSE.
- Oeller, M., et al., Selection of Tissue Factor-Deficient Cell Transplants as a Novel Strategy for Improving Hemocompatibility of Human Bone Marrow Stromal Cells. Theranostics, 2018. 8(5): p. 1421-1434. 10.7150/thno.21906
