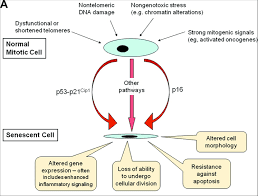
 |
Extreme cellular stress can trigger senescence, a mechanism protecting
against malignant cell transformation.
Adapted from: Kovacic J C et al. Circulation. 2011;123:1650-1660 |
We’ve mentioned several times on this blog how standardization of materials, equipment, and processes is critical to driving reproducibility and robustness of living cell technologies. As we continue to engage researchers in Industry and Academia in high-level scientific discussions, it has become apparent that we, as a community, need to adopt more standardized means of accounting for our cell culture practices and what that really means in terms of Mesenchymal Stem Cell (MSC) age and function.
Given
MSCs definition as the plastic-adherent fraction of the bone marrow, and their need to be expanded ex vivo prior to therapeutic administration, age has often been associated with and reported as a function of passage level, or the number of times these cells have been plated onto and harvested from tissue culture plastic. True MSC age, however, is actually related to when these cells will senesce (i.e. stop growing), and this is a function of how many times the cells have divided, or their population doubling level (PDL).
The
World Health Organization, in their “Recommendations for the evaluation of animal cell cultures as substrates for the manufacture of biological medicinal products and for the characterization of cell banks” defines
in vitro cell age as the “Measure of time between thaw of the Master Cell Bank vial(s) to harvest of the production vessel measured by elapsed chronological time, by population doubling level of the cells, or by passage level of the cells
when subcultivated by a defined procedure for dilution of the culture“.
Thus, without knowledge of the defined procedure for subcultivation, passage number is a subjective measure of cell age.
In addition, the
MSC Reference Materials Group, a group of MSC experts attempting to generate cell-based reference materials to advance MSC research and clinical translation, recommends that, for standardization of the field, “…the protocol for preparation of MSCs could be documented in cells/sq cm at plating and at harvest, and by population doublings per passage”.
Finally, the recently published
FDA perspective on MSC-based product characterization for clinical trials states: “Although population doubling data is more informative [than passage level data], it is often not described”.In the spirit of standardization and transparency, we report the PDL of each released lot of MSCs that is calculated from the time the first adherent Mononuclear Cell population has been harvested. We believe that this best practice helps us communicate with our customers and the field using a standardized nomenclature that can be used across laboratories. This information, coupled with donor age, gives a close approximation to the lifespan of the MSC. It is, of course, not perfect, but it is much more accurate than passage level – in our opinion.
Passage level, at the end of the day, is too variable of a metric to provide insight into the age of the cells in question, so why are we still reporting MSC age this way? Let’s start talking about “real MSC age”, their population doubling level, and begin comparing apples to apples….
What are your thoughts on reporting passage level vs PDL? Would reporting cell seeding and harvest densities, doubling times, and PDLs do more to advance the field?
RoosterBio is fueling the rapid implementation of scalable advanced therapies. Contact us to discuss how we can accelerate your product & process development. Follow us on LinkedIn for more educational resources just like this.

