“You mean, sit on top of a rocket and launched into space? Sounds dangerous; when do we go?”
The Right Stuff (1983)
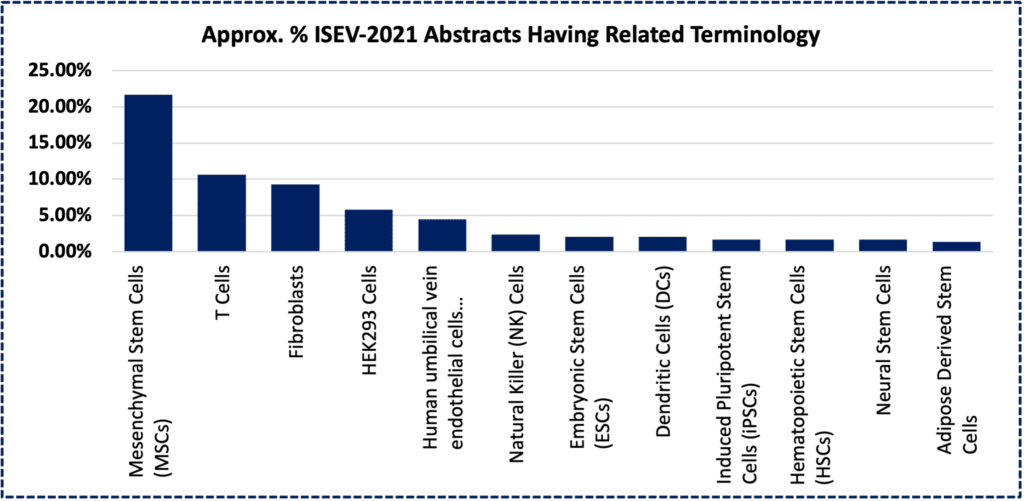
Above, bar chart depicting estimated fraction of >500 abstract/titles from the annual 2021 meeting of the International Society for Extracellular Vesicles (ISEV), containing keywords related to a specific primary (or transformed- HEK293) cell type. [1]
Over the last several years, we’re increasingly asked a version of this question: Why do regenmed clinical product developers look to human mesenchymal stromal/stem cells (hMSCs) as producer cells for therapeutic extracellular vesicles (EVs)? Despite growing exuberance surrounding EVs (or their related terminology -i.e., “exosomes”) as a human therapeutic delivery system, there are many choices of cell type that could be available to suit this purpose. We recognize that sources like our immune system’s dendritic cells (i.e., yielding “dexosomes”), T-cells (via CAR-T), and NK cells (NK-92), or other cultured cells such as HEK-293, fibroblasts (e.g., HFFs), iPSCs—or even products like cow’s milk—might serve unique roles in this opportunity space. Yet it’s useful to circle back on the question of “How might MSC-EVs have the ‘Right Stuff’?” The short answer is simple. Not unlike early Mercury Program astronauts, some hMSCs and their corresponding EV biomaterials have already passed through a protracted gauntlet of quality controls, all intended to minimize the risk of preclinical or clinical study failure. At high level, risks that EVs encounter along a clinical development path are categorized twofold: (1) bioproduction and (2) clinical. We will address them both in this brief blog.
hMSCs are currently the second most-used “stem” cell source in human clinical cellular therapeutics,[2] ranking only behind HSCs (hematopoietic stem cells).[3] While evidently not a true “stem cell” in terms of differentiation potential,[4] these workhorse progenitor cells do exhibit an extended replicative capacity and can be mass cultured under optimized GMP conditions, to meet the needs of thousands of trial patients, each year.[5, 6] Steady process innovations are underway. These aim to reduce costs and improve quality of hMSCs across key metrics, and are now becoming more standard.[7, 8, 9, 10, 11] Examples of key readouts are total costs and liters of media to yield a billion cells under cGMP conditions, identity markers to be maintained uniformly across 2D and 3D biomanufacturing runs, and initial tissue donor parameters to expand cells. MSCs are also routinely adaptable to exhibit consistent and rapid doubling times in serum-free media, with IND reference-ready Type II FDA Master Files.
Yet while hMSCs are hardy and robust, even larger (100L+) volume production scales will be needed to minimize production costs such that MSCs can finally be lauded as the “CHO Cell” of regenerative medicine.[12] Nevertheless, MSCs as a cell class are also known to be among the most prolific natural secretors of EVs, even under non-stimulated, basal conditions.[13, 14] In addition, artificial physical, chemical, and genetic process manipulations of hMSCs to increase EV yields are an area of active and rapid innovation.[15] Such mass-produced MSC-EVs can now be yielded through standard harvesting procedures to display the same basic size distribution, molecular markers, morphological features, and bioactivities.[16] For these reasons and others, MSCs are often a “go-to” cell system for EV production, as evidenced by a strong plurality presence in the abstract books at conferences like ISEV-2021 (see chart at top of page).[1] At finer resolution, however, heterogeneity does happen, even with MSC-EVs.[17, 18]
Lessons learned from the HEK-293T bioproduction platform vis-à-vis lentivirus, AAV, and adenovirus gene therapy vectors or vaccines will certainly apply to EVs, which may share some uncannily similar vestigial features with retroviruses/lentiviruses.[19] Fortunately, much like “old school” cell lines, hMSCs can be readily genome edited or otherwise modified with transgenes and/or priming treatments.[20, 21] These molecular toolkits enable their engineered cells’ final secreted product to be more uniformly produced via “built-in” control circuitry embedded in the cell nucleus—like software apps. Such breakthroughs will surely propel many future MSC-EV products through the necessary safety and efficacy endpoints.[22]
GMP-produced EVs/exosomes from dendritic cells (“dexosomes”) were first used in human therapies to vaccinate against tumors in the early-2000s at ISERM, with Anosys.[23, 24] This pioneering work soon cross-pollinated an effort to see if a parallel mechanism could suppress the immune system in corticosteroid-resistant GvHD, via MSC-EVs. The first compassionate use study (2011) with a patient at the U. of Duisburg-Essen suggested safety, and likely a signal of a least some months’ duration of relief from the brutal GI and dermal symptoms.[25] The Duisburg-Essen study—and a flurry of preclinical studies across multiple indications and centers of expertise through the 2010s—provided credible evidence for an alternative mechanism for therapeutic hMSCs. That is, many MSC preparations seemed to work best not via direct engraftment into (and regeneration of) damaged tissue,[26] but rather through a paracrine secreted product like EVs.[27] Meanwhile, tens of thousands of patients have now safely been administered MSCs across hundreds of registered clinical trials, worldwide.[28] Many of these studies involved allogeneic cells, a long-established bioproduction advantage for MSCs facilitated by their very low immunogenicity.[29] The idea that some MSC patients may not have needed fastidious preparations of injected living cells (rather instead, only cell “secretomes”) is surely eye-opening, and beckons an opportunity in regenmed technology.[30] Accordingly, academic and/or translational centers such as MD Anderson Cancer Center (e.g., NCT03608631, KRas-driven pancreatic cancer),[31] Jiao Tong University (NCT04388982, Alzheimer’s; NCT04602104, ARDS; NCT04276987, COVID-19),[32, 33, 34] Tianjin Medical University Hospital (NCT03437759, macular holes),[35] Children’s Hospital of Fudan University (NCT04850469, severe infections),[36] and the Royan Institute (NCT04366063, COVID-19 ARDS),[37] and also commercial entities like United Therapeutics (NCT03857841, Bronchopulmonary Dysplasia in neonates),[38] Aegle Therapeutics (NCT04173650, Epidermolysis Bullosa),[39] Direct Biologics (NCT04493242, severe COVID-19)[40] now pursue clinical trials using MSC-EVs. Other MSC-EV programs in preclinical stages are directed across a span of indications as broad the original MSC cellular therapies.[41, 42, 43]
It’s nearly self-evident why MSC-EVs could edge in front of many MSC therapies now currently administered as living cells. First, the EV product can be sterilized by filtration, greatly reducing the risk of contamination, and perhaps allowing for fewer constraints on clinical grade clean rooms. Second, although EVs can (and will) be engineered to carry genetic or genetically modified therapeutic molecular cargoes, EVs do not self-replicate like viruses or cells, and do not themselves elicit an intrinsic tumor forming activity. (Unlike known CAR-T or HSC-based therapies, for example, EV from MSCs would not need a “suicide switch.”) Third, although heterogeneity remains a point of contention at this early stage, MSC-EV processes, batches, and critical quality attributes (CQAs) could certainly be defined and managed more uniformly than for their corresponding parental cells. Fourth, EVs may be much easier to handle than live cells, apparently robustly withstanding freeze/thaw, storage, and bedside injection parameters; these biomaterials (vs. cells) would demand less training of clinic personnel. “Why bother buying the cow when you can directly buy the milk” is a widely expressed analogy.[44] Finally—although there’s more effort required to reach this objective—EVs will one day be more scalable to economically produce higher dose numbers per patient than direct cellular therapeutics.
In conclusion, EVs from MSCs are promising because these liposome-like “drug delivery particles” originate from a bioproduction source that is easy to grow via off-the-shelf, and standardized cGMP-compliant ancillary and starting materials. hMSCs as a bio-source provide for the some of the most widely used experimental allogeneic cellular therapies, having earned a track record with over two decades of safety. Although the precise mechanisms of action are still thoroughly investigated (and debated), it does appear that a basic repertoire of molecular factors from these MSCs can be sufficiently transferred via EVs, collected out of cultured supernatants from an industrial bioreactor. At institutions like RoosterBio, growing expertise to optimize scalable cGMP hMSC manufacturing, infrastructure, bioprocess, and novel EV production platforms for industrial quantities of MSC-EVs is being leveraged today. As we look to the future of this exciting field, it will surely involve engineering of MSC-EV therapeutic effector cargos, in vivo targeting, and molecular control circuits to override clinical variability in both product quality and performance. In a word, MSC-EVs do seem to have the Right Stuff, not just for biotechnology’s equivalent of John Glenn’s Friendship 7, but also for regenmed’s future SpaceX Dragon.
References
- ISEV2021 Abstract Book. Journal of Extracellular Vesicles, 2021. 10(S1): p. e12083. https://doi.org/10.1002/jev2.12083
- clinicaltrials.gov. Web query to clinicaltrials.gov – “mesenchymal stem cell” [interventional]. 2021; Available from: https://bit.ly/3dmC8Ds.
- clinicaltrials.gov. Web query to clinicaltrials.gov – “hematopoietic stem cell” [interventional]. 2021; Available from: https://bit.ly/3y6GAy3.
- Caplan, A. I., Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med, 2017. 6(6): p. 1445-1451. 10.1002/sctm.17-0051
- Caplan, A. I. and D. Correa, The MSC: an injury drugstore. Cell Stem Cell, 2011. 9(1): p. 11-5. 10.1016/j.stem.2011.06.008
- Rowley, J.; Farrance, I, Carson, J. What Are MSCs? 2020; Available from: https://www.roosterbio.com/blog/what-are-mscs/.
- Campbell, A., et al., Concise Review: Process Development Considerations for Cell Therapy. Stem Cells Transl Med, 2015. 4(10): p. 1155-63. 10.5966/sctm.2014-0294
- Ahsan, T.; Adlerz, K. Best Practices in MSC Culture: Tracking & Reporting Cellular Age Using Population Doubling Level (PDL) & Not Passage Number. 2019; Available from: https://bit.ly/3dmFINL.
- Lembong, J., et al., Bioreactor Parameters for Microcarrier-Based Human MSC Expansion under Xeno-Free Conditions in a Vertical-Wheel System. Bioengineering (Basel), 2020. 7(3). 10.3390/bioengineering7030073
- Olsen, T. R. and J. A. Rowley, Corporate profile: RoosterBio, Inc. Regen Med, 2018. 13(7): p. 753-757. 10.2217/rme-2018-0092
- Rowley, J.; Carson, J. Adherent Cell Manufacturing’s Call to Action: Manufacturing Platform & Media Strategy Matters for Mesenchymal Stromal/Stem Cell Production Economics. RoosterBio Blog 2021; Available from: https://bit.ly/2UDvHFi.
- Olsen, T. R., et al., Peak MSC-Are We There Yet? Front Med (Lausanne), 2018. 5: p. 178. 10.3389/fmed.2018.00178
- Yeo, R. W., et al., Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev, 2013. 65(3): p. 336-41. 10.1016/j.addr.2012.07.001
- Adlerz, K., et al., Scalable manufacturing system for MSC-EV generation. Cytotherapy, 2020. 22(5, Supplement): p. S46. https://doi.org/10.1016/j.jcyt.2020.03.051
- Adlerz, K., et al., Increasing yield of msc-evs in scalable xeno-free manufacturing. Cytotherapy, 2019. 21(5, Supplement): p. S58. https://doi.org/10.1016/j.jcyt.2019.03.433
- Adlerz, K., et al., Comparison of msc-evs manufatured in 2D versus scalable 3D bioreactor systems. Cytotherapy, 2019. 21(5, Supplement): p. S58. https://doi.org/10.1016/j.jcyt.2019.03.434
- Patel, D. B., et al., Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment. Biotechnol Adv, 2018. 36(8): p. 2051-2059. 10.1016/j.biotechadv.2018.09.001
- Kalluri, R. and V. S. LeBleu, The biology, function, and biomedical applications of exosomes. Science, 2020. 367(6478). 10.1126/science.aau6977
- Gould, S. J., A. M. Booth, and J. E. Hildreth, The Trojan exosome hypothesis. Proc Natl Acad Sci U S A, 2003. 100(19): p. 10592-7. 10.1073/pnas.1831413100
- Willstaedt, T. Genetically Engineered Mesenchymal Stromal Cells: A Promising Tool for the Successful Delivery of Therapeutic Genes. RoosterBio Blog 2021; Available from: https://bit.ly/3jp6ybY.
- Carson, J. eBook: Genome Editing of MSCs. 2021; Available from: https://www.roosterbio.com/blog/ebook-genome-editing-of-mscs/.
- Krawczyk, K., et al., Rewiring of endogenous signaling pathways to genomic targets for therapeutic cell reprogramming. Nat Commun, 2020. 11(1): p. 608. 10.1038/s41467-020-14397-8
- Le Pecq, J. B., Dexosomes as a therapeutic cancer vaccine: from bench to bedside. Blood Cells Mol Dis, 2005. 35(2): p. 129-35. 10.1016/j.bcmd.2005.06.003
- Lamparski, H. G., et al., Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods, 2002. 270(2): p. 211-26. 10.1016/s0022-1759(02)00330-7
- Kordelas, L., et al., MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia, 2014. 28(4): p. 970-3. 10.1038/leu.2014.41
- Gnecchi, M., et al., Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol Biol, 2016. 1416: p. 123-46. 10.1007/978-1-4939-3584-0_7
- Eleuteri, S. and A. Fierabracci, Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int J Mol Sci, 2019. 20(18). 10.3390/ijms20184597
- celltrials.org. Cell Trials Data. celltrials.org 2021; Available from: https://celltrials.org/cells-data
- Elahi, F. M., et al., Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells, 2020. 38(1): p. 15-21. 10.1002/stem.3061
- Forsberg, M. H., et al., Mesenchymal Stromal Cells and Exosomes: Progress and Challenges. Front Cell Dev Biol, 2020. 8: p. 665. 10.3389/fcell.2020.00665
- Center, M. D. Anderson Cancer, iExosomes in Treating Participants With Metastatic Pancreas Cancer With KrasG12D Mutation. 2022.
- Ruijin, Hospital and Ltd Cellular Biomedicine Group, the Safety and the Efficacy Evaluation of Allogenic Adipose MSC-Exos in Patients With Alzheimer’s Disease. 2021.
- Ruijin, Hospital and Ltd Cellular Biomedicine Group, A Clinical Study of Mesenchymal Stem Cell Exosomes Nebulizer for the Treatment of ARDS. 2022.
- Ruijin, Hospital, et al., A Pilot Clinical Study on Inhalation of Mesenchymal Stem Cells Exosomes Treating Severe Novel Coronavirus Pneumonia. 2020.
- Tianjin Medical, University, MSC-Exos Promote Healing of MHs. 2018.
- Children’s Hospital of Fudan, University. Study of MSC-Exo on the Therapy for Intensively Ill Children. 2023 December 31; Available from: https://ClinicalTrials.gov/show/NCT04850469.
- Royan, Institute, Sciences Tehran University of Medical, and Sciences Shahid Beheshti University of Medical, Mesenchymal Stem Cell Therapy for SARS-CoV-2-related Acute Respiratory Distress Syndrome. 2020.
- United, Therapeutics, A Safety Study of IV Stem Cell-derived Extracellular Vesicles (UNEX-42) in Preterm Neonates at High Risk for BPD. 2021.
- Aegle, Therapeutics, MSC EVs in Dystrophic Epidermolysis Bullosa. 2023.
- Direct Biologics, L. L. C., Extracellular Vesicle Infusion Therapy for Severe COVID-19. 2020.
- Tsiapalis, D. and L. O’Driscoll, Mesenchymal Stem Cell Derived Extracellular Vesicles for Tissue Engineering and Regenerative Medicine Applications. Cells, 2020. 9(4). 10.3390/cells9040991
- Shekari, F., et al., Pre-clinical investigation of mesenchymal stromal cell-derived extracellular vesicles: a systematic review. Cytotherapy, 2021. 23(4): p. 277-284. 10.1016/j.jcyt.2020.12.009
- Exosomes – Pipeline Insight, 2021. 2021; Available from: https://bit.ly/3qZS6IX.
- Sharma, D. and F. Zhao, Updates on clinical trials evaluating the regenerative potential of allogenic mesenchymal stem cells in COVID-19. NPJ Regen Med, 2021. 6(1): p. 37. 10.1038/s41536-021-00147-x
