Prolific Stem Cell and Cell Therapy Blogger Alexey Bersenev (@cells_nnm on Twitter) has recently written a post on his Cell Trials blog titled “Trends in cell therapy clinical trials 2011 – 2013”. If you are interested in Stem Cells and Cell Therapy, it is highly likely that you already know Alexey. He is one of the most passionate and dedicated technologists in the area. If you don’t know him, you can follow his activities at the above links, as well as at his Stem Cell Assays blog and among several LinkedIn Discussion groups. Alexey reported that his own research shows that Cell Therapy Clinical Trials that are listed in international databases have doubled from 2011 to 2013, going from 161 to 324. Alexey evaluated the data by the split between industry and academia (three academic trials in 2013 for every industry-sponsored trial), by country, by region, as well as by cell type and clinical indication. His analysis is very important, because, with his technical background and expertise (Alexey is an MD/Ph.D. researcher who has been doing hands stem cell research or therapeutic cell manufacturing as his day job for several years), if anyone can correctly categorize a trial, it is Alexey.
When tweeting highlights of his Cell Therapy Clinical Trials blog post (above inset) an astute follower of his picked up on the fact that Mesenchymal Stem/Stromal Cell (MSC) trials are out-pacing the other cell types. This fact is consistent with another often-cited recent manuscript printed in the journal Regenerative Medicine titled “The Global Landscape of Stem Cell Clinical Trials” (free download available here). This manuscript also creates its own database and analyzes the various types of trials, with the conclusion that “most of the increase (in Cell Therapy Clinical Trials) since 2006 was due to trials using MSCs”.
So WHY are MSC trials outpacing other cell sources? There are several reasons for this. We believe this is likely due mostly to:
- MSC researchers enjoy a tremendous data set generated over the last 20 years, and the safety profile of MSCs in early-stage clinical trials has been very good. This accumulated data has created a more straightforward regulatory path with MSCs when compared to many other cell types such as pluripotent stem cell (PSC)-derived therapies. PSCs are still in their infancy from the clinical trial side, and the next 10 years should considerably de-risk these cells.
- Many MSC trials are performed with undifferentiated cells, and the manufacturing processes are simpler, more standardized, and more robust than many differentiated cell products. This leads to more straightforward CMC (Chemistry, Manufacturing, and Controls) filings when compared to newer cell types that have complicated differentiation pathways that have never been scaled up. The standardization over the last several years has led to greater robustness, with more consistency around the cost of goods.
- Finally, due to their robust signaling activity, MSCs demonstrate usefulness in a wide range of clinical indications (see Figure below borrowed from Viswanathan et al., Stem Cells Dev. 2014, Jan 14) including cardiac, vascular, neurological, and various orthopaedic regenerative applications, not to mention immunological applications (eg. GVHD) and more recently cancer therapies. MSCs can be used alone or with other cell types to facilitate engraftment of these other cells by modulating host immune response to “protect” these cells. Since a single manufacturing process can produce MSCs for use in a variety of clinical trials, the clinical application of these cells is accelerated compared to other cell types.
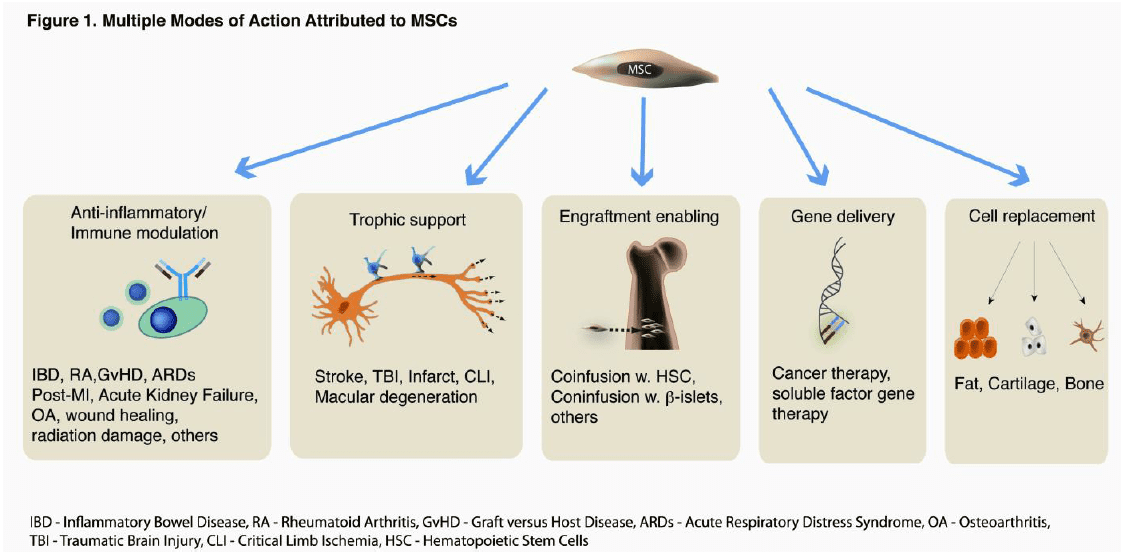 |
MSCs truly are the Workhorse of Regenerative Medicine, and their use in Orthopaedic applications is where clinical translation was initially imagined. However, the robust signaling activity of MSCs has widened the range of clinical indications to include cardiac, vascular, and neurological regeneration, as well as immunological applications (eg. GVHD, Chron’s disease), and more recently, cancer therapies.
It is thus not surprising that MSCs were a strong component of the annual meeting of the Orthopaedic Research Society in New Orleans, LA in March. We were fortunate enough to attend this conference and see the vast amount of work being conducted in the orthopaedic tissue engineering arena. Not surprisingly, there were many presentations on the use of MSCs for musculoskeletal tissue repair and regeneration. Below is some of the MSC research that caught our eye.
Tissue Engineered Periosteum Approaches to Heal Bone Allograft Transplants
- Michael Hoffman, Benoit Lab, University of Rochester
- Transplantation of decellularized bone allografts seeded with both undifferentiated MSCs and MSCs differentiated to the osteogenic lineage lead to modulation of VEGF production and an increase in BMP2 production that resulted in an increase in torsional biomechanical graft host stability and rate of endochondral ossification compared to allograft alone and allograft seeded with undifferentiated MSCs alone.
- A related publication can be found here.
Anatomically Shaped, Vascularized Bone Grafts for Craniomaxillofacial Reconstruction
- Joshua Temple, Grayson Lab, Johns Hopkins University
- 3D printed porous scaffolds with correct anatomical features were successfully created to regenerate complex craniofacial deformities. Scaffolds containing adipose MSC aggregates formed extensive vascular networks as well as bone. Implanted scaffolds demonstrated patent human vasculature and bone formation.
- A related publication can be found here.
Anatomic Hypertrophic Cartilaginous Grafts For Whole Bone Tissue Engineering
- Eamon Sheehy, Kelly Lab, Trinity College, Dublin
- Alginate, chitosan, and fibrin hydrogels seeded with MSCs were investigated for bone formation through endochondral ossification. While all constructs underwent robust chondrogenesis in vitro, alginate supported the greatest degree of endochondral bone formation in vivo, with the establishment of a hematopoietic marrow component with evidence of blood vessel infiltration. Subsequently, anatomically correct, MSC-seeded alginate hydrogels were used as the osseous layer of an engineered phalanx construct, which successfully underwent spatially regulated endochondral ossification in vivo.
- A related publication can be found here.
Inherent and Emergent Heterogeneity in Clonal Stem Cell Populations
- Brian Cosgrove, Mauck Lab, University of Pennsylvania
- Clonal MSC populations were compared to mixed, or non-selected MSC populations in terms of inherent heterogeneity. Surprisingly, extensive biophysical heterogeneity exists both between clones and within clones. This heterogeneity emerges very early in colony formation, persists in culture, and is amplified with the addition of soluble factors to the culture medium. Thus, heterogeneity in MSC populations has both inherent and emergent components. Better understanding MSC heterogeneity and when it emerges could result in improved methods for successful MSC differentiation.
In Vivo Function and In Vitro Characterization of Tissue Engineered Intervertebral Discs Made with Human Mesenchymal Stem Cells
- Katherine Hudson, Bonassar Lab, Cornell University
- Tissue-engineered intervertebral discs were made using alginate and collagen seeded with MSCs to recreate the nucleus pulpous and annulus fibrosus. In vitro, TE-IVDs treated with chondrogenic differentiation medium demonstrated increased stiffness and ability to pressurize compared to controls maintained in growth medium. In vivo, MSC-containing discs maintained more hydration that punctured and degenerate discs. In addition, MSC seeded discs maintained disc height for two weeks in vivo.
- A related publication can be found here.
One of the highlights of the conference was the special session dedicated to and titled “Mesenchymal Stem Cells”. Frank Barry from Galway highlighted this session with a great overview of the MSC field. It seemed like this was by far the most heavily attended session at the conference and the take-home message from the standing room only attendance is that there is tremendous interest in and promise of MSC use in orthopaedic research and development.
Clearly, MSC research in the orthopaedic space spans from understanding the basic mechanisms of MSC growth and differentiation to various application modalities of MSCs to treat a number of traumatic injuries and diseased states. Findings presented at the ORS meeting demonstrated that MSCs aid in tissue repair via differentiation to intended lineages, through secretion of paracrine factors and through neovascularization. We were very excited to see the emergence of 3D printing technologies incorporating MSCs from Hopkins, Cornell, and Utrecht (where you can now get a Master’s degree in Bioprinting!), and we look forward to continued discussion on understanding MSC heterogeneity and methods to better standardize MSC culture and clinical applications for Regenerative Medicine.
Keep an eye out for a future post on research presented at the Society for Biomaterials meeting that took place in Denver, CO.
