Listen to this Blog:
To many biologists and immunologists, the notion that T cells and mesenchymal stromal/stem cells (MSCs) can be compared in the same “ice bucket” sounds, at best, simplistic. Nevertheless, both cell types are indeed routinely measured against each other by regenerative medicine product developers as they “whiteboard” their early concepts and TPPs. As the basis for emerging technology and therapeutic platforms, these two distinct lineages each have unique talents that can be aimed at both overlapping and discrete unmet medical needs. Even more fascinating is how T cells and MSCs don’t have to compete for program time and resources—but can instead work together quite nicely!
T Cells Versus MSCs?
T cells are sometimes considered for adoptive transfers of tumor infiltrating lymphocytes (TILs), [1] or they’re mined for cloned T cell receptors that target neoantigens. [2] The most advanced therapeutic format for T cells is CAR-T (Chimeric Antigen Receptor) therapies, [3, 4, 5, 6, 7] however, with five approved by the FDA as of this writing (2022). [8] Today’s approved CAR-Ts are based on ex vivo expansions from autologously derived cells, which have initially involved an onerous off-site manufacturing process [9] that’s difficult to scale, lasting 3+ weeks. [10] In theory, anything to simplify the drug process such as pre-banked allogeneic CAR-T cells could be a game changer. [11, 12] In practice, “allo” CAR-T can ignite a graft versus host disease (GvHD) where the donor T cells’ endogenous T cell receptor can attack the patient’s own tissues; also, absent any powerful immunosuppressive regimens, the host’s immune system can attack the donor CAR-T cells.
Immunologic incompatibility with “allo-CARTs” can be remedied by gene editing to delete the CAR-T’s native T cell receptor, [13] or to select for T cells from clones that once battled a universally common virus (e.g., Epstein-Barr Virus, EBV). [14] A different approach to minimize the bioprocess complexity is to “stay autologous” by forward engineering the CAR-Ts with built-in genetic tweaks, such that weeks-long expansion steps in bags are eliminated after one patient blood draw. Only thawing, gene transfer, and re-animation could be needed, which would shorten one’s hospital stay to a single visit of 1-2 nights. These genetic modifications empower the CAR-T to expand not ex vivo, but rather in patient, under the control of cytokine signaling like from IL-15. [15]
Unlike many T-cells, the main job description for MSCs isn’t killing virus-infected cells but seems related to tissue repair—and resolution of a hypoxic and inflammatory microenvironment after infection clearance. [16, 17, 18] Like T-cells, MSCs have been in clinical trials for decades, but MSCs can be safely administered as an allogeneic therapy with no prior immunosuppression. [19] In fact, they appear to be tolerogenic, showing signals of clinical efficacy against diseases like GvHD or autoimmune indications like arthritis. [20] Lower hurdles toward allogeneic approaches with the MSCs facilitated their bioreactor expansion technologies [21] to accommodate higher cell numbers than T cells by two orders of magnitude. These instrument systems are now demonstrated to exceed 100-liter batch volumes [22] and cell counts in the 10s of billions [23] —enough doses for 10s of trial patients per run. [24, 25, 26] One possible issue with MSCs is that they have not yet been greeted with an approval from the FDA after many years, despite consistently good safety and reams of data showing efficacy in preclinical studies. Even though at least 15 MSC-related products have been granted regulatory approval outside of the USA, an analog of 2017’s Kymriah and Yescarta breakthrough event with MSCs is still around the corner.
For MSCs to be anointed with a “Kymriah moment” within the USA, allo-MSCs may need to be manufactured and administered even more consistently and with stringent control of the CQAs and CPPs, applying an industrial mentality to right-scaled applications. [27, 28, 29, 30] That’s how some of the observed variations in dose potency can vanish—and deliver on the primary endpoints (p<0.05) for late-phase trials. And, to support indications that require 100s of 1000s of annual doses, MSCs will need a clear manufacturing path for growth in bioreactors at even higher volume scales (e.g., 1000L+). The Cell Manufacturing Consortium in alliance with NIIMBL has been at the forefront of this ongoing effort, in which RoosterBio has played a noted role.
In addition to thinking of “downstream” process improvements, allo-MSCs can also be greatly enhanced “upstream” with multi-gene modifications, just like CAR-Ts. [31, 32] This way, the MSC becomes the controlled delivery system of a programmable genetic medicine, not simply a cell medicine. Incidentally, MSCs can even be programmed to express chimeric antigen receptors that target them to (and/or retain them within) tumors, which can then be attacked at close range with molecules like TRAIL, or perhaps with prodrug convertases like HSV-TK. [33, 34] In addition, MSCs can be manipulated metabolically or morphologically by changing media/oxygenation or growth substate, respectively, decreasing their dose-to-dose heterogeneity and fine-tuning their potency towards specific indications. [18, 35] Finally, as others have said, “Why bother buying the cow when you can directly buy the milk?” [36] MSCs are prolific producers of therapeutic secretome products like EVs/exosomes or perhaps even mitochondria, and cell engineering efforts are underway to enhance MSCs to yield fully primed regenmed pipelines via a variety of platform technologies. [37, 38]
Of course, T cells can and do perform some of MSCs’ “tricks” as well. MSCs—known for their immunosuppression—often work as a team with regulatory T cells (Tregs) and other cells in the body. [39, 40] Adoptive-transferred, autologous Tregs to combat inflammatory diseases was advanced over a decade ago by Dr. Zelig Eshhar (aka the inventor of CAR-T) and others. [41, 42, 43] Some of these concepts advanced into human clinical trials, [44, 45, 46] with signs of possible efficacy. Tregs can likewise be re-targeted against specific autoantigens by CARs (i.e., CAR-Tregs). [47, 48, 49, 50] With Tregs as relatively rare white blood cells, the challenge of obtaining pure populations can be lessened by differentiating them artificially via Foxp3 overexpression in T cells, [51] or culture in media containing TGF-b or elevated IL-2.
So, neither T cells nor MSCs “win the gold medal” in the overall contest of who’s better! (But we knew that already, didn’t we?) The outlook for 2022 and beyond for both T cells and MSCs remains bright, although clearly there’s much that proponents of each can learn from one another. For starters, the cost per dose must be dramatically reduced. Hence, what’s been termed “Life Science Industrials” by Dynamk Capital is sure to play an important upcoming role for the pioneers of CAR-T and MSCs that demand a solid bridge between cell-drug discovery and biomanufacturing.
| Cell Type | Autologous Uses | Allogenic Uses | Genetic Modification | Industrial Scalability | Versatility for Clinical Indications | Bio-production of Tx Products | Regulatory Approvals |
| T Cells | ++ genetic engineered T cells can bring dramatic remissions in blood cancers, aimed at the appropriate indications; ongoing breakthroughs may yield fewer processing steps in less time between leukapheresis & re-infusion | + “allo” T cells can elicit serious side effects without genome editing tweaks and/or selection of natural EBV-restricted clones (now underway); “rejuvenated” T cells expanded through an iPSC phase might offer unlimited supply if properly engineered | ++ mostly CAR-T gene products and related edits & integrations to improve potency, on-target selectivity, and manufacturability | + up to 100s of millions of cells via Wave bags for a single autologous patient dose | + highly effective for oncology; Tregs (including CAR-Tregs) might be next for auto-immune indications; possibly CARs vs infectious disease or fibrosis? | -/+ academic study of T cell EVs | +++ multiple CAR-T for liquid tumors |
| hMSCs | ++ autologous hMSCs can be available in great abundance from lipoplasty surgeries via “adipose stromal cells — less invasive than bone marrow and easier to isolate than from cord tissue | +++ hMSCs are long known for a tolerogenic effect, hence likely utility to treat GVHD; safely administered as “allo” in 800+ clinical trials since 2011 | +++ diverse gene products integrated or edited into hMSCs as therapeutic cargoes or manufacturing enhancers; a few are now in clinical trials | +++ > tens of billions of cells per bioreactor run possible at 50L scale, for dozens of patient doses | +++ studies in progress for diverse regenmed indications including oncology, GvHD and COVID/ARDS; “CAR-MSCs” demo’d in rodents | +++ EVs/exosomes from MSCs are in ongoing clinical trials; MSC-derived conditioned media or mitochondria are of growing interest | -/+ outside of USA only |
Table, above: T Cells and MSCs currently differ as cell and gene therapy platforms across different parameters (autologous and allogeneic uses, genetic modification, industrial scalability, versatility for clinical indications, use as bioproduction systems for secreted cell products, and status of clinical approval). Perhaps these differences can create opportunities for experts of both cell types?
T Cells Via MSCs!
In the battles against diseases like cancer, we have elite commandos (T cells) to call on. But MSCs are not “commandos”—nor are they as awe-inspiring as the cardiomyocytes that pump our hearts—or the neurons that keep us breathing, moving, and thinking. But if combatting disease, shouldn’t we recruit a few good medics? [52] It turns out that T cells and MSCs are “blood brothers” in arms throughout the animal kingdom, emerging together from the smoke of every fight with a pathogen or an acute injury. MSCs can also exert some anti-microbial activity, [53] but they’re also around to help repair the carnage after a disease waves its white flag. [17] Why not harness this special relationship therapeutically?
In physiology, T cells rely much on dendritic cells (DCs) as professional antigen presenting cells (APCs) to cross-present processed foreign peptides for display on MHC molecules. These T cells then expand in response to the specific peptide signal that is a flag of “non-self.” However, unlike MSCs, DCs are not necessarily easy to grow and process at industrial levels of scale. Work by the lab of Dr. Moutih Rafei of McGill University has shown that MSCs can be chemically re-polarized from an immunosuppressive state into one that is immune-stimulating—and targetable to specific antigens for presentation to T cells, in APC-like fashion. [54] This new technology could potentially enable T cells (like CAR-T) to selectively expand via co-culture with “MSC-APC” material, enriching their potency in a target-definable manner. MSC material that is genetically modified and primed in this way could also possibly serve in a vaccine candidate screening or manufacture platform. In a parallel use of MSCs to stimulate T cell killing, MSCs can be modified to secrete bi-specific antibody fragments (e.g., analogous to “BiTEs”) that induce T cells to junction with and lyse specific tumor cells that bear the target antigen. [55] Or, as homing cells, they could prime a tumor microenvironment for CAR-T killing by ectopic expression of pro-Th1 cytokines. [56] What might happen when CAR-T are added in vivo along with MSC? Do MSCs suppress the CAR-T benefit, or (perhaps?) only the side effects of a raging tumor lysis syndrome? Zanetti, et al (2020) showed that MSCs isolated from pediatric leukemia patients could still actively suppress a model of severe colitis in mice, and yet did not suppress the anti-tumor activity of a CD19 CAR against in vivo NALM6 cells. [57] Given that CAR-Ts can be accompanied by serious side effects, it would be interesting to observe whether co-transplanted MSCs can improve patient quality of life and recovery.
Although CD8+ T cells are notorious “serial killers”—something we want when battling cancer—they can overzealously leave behind much “collateral damage” and potentiate adverse remodeling after heart attack. When co-cultured, MSCs reduce T cell proliferation through both contact-dependent and -independent means, including via EVs/exosomes. [58] This suggests a possible avenue for screening for the ideal engineered MSC-EV candidate vs. T cells as a therapeutic to use against acute cardiac indications. Other potential examples of application include re-education of polarized T cells via MSCs to reverse rheumatoid arthritis, [59] multiple sclerosis, [60] idiopathic pneumonia syndrome, [61] or other diseases. Yet the benefits of MSCs to Tregs in an immunosuppressive therapy are not all “one-way;” in vivo depletion of Tregs adversely affects both MSCs and HSCs, perhaps due to scarcity of IL-10 mediated signaling in certain contexts. [39]
MSCs are increasingly administered in human clinical trials for traumatic brain injury (TBI), such as via the group of Dr. Charles Cox of the University of Texas Health Sciences Center. Pathologic neuroinflammation is a hallmark of TBI, and recent animal studies show how Tregs and MSCs could together yield a potent super-additive benefit. [62] Interestingly, timing of infusion may also be critical to observe positive effects, where it may be helpful to first inject the Tregs, and second, the MSCs. Although the precise mechanism seems due to reduced inflammation and not necessarily blood brain barrier (BBB) repair, [63] we eagerly await the possibility to see how they could perform together in some of the more than 1.5M yearly patients in the USA, alone.
One potential application for MSCs in T cell therapies (e.g., Tregs) is to co-culture them before reimplantation as a bioprocessing accessory to T cells—albeit not a constituent of the final cell-drug. To this author’s knowledge, this direction has not yet been clinically tried with T cells, but rather, with another key immunological cornerstone: monocytes. In a recent “first,” the group of Dr. Ashish Patel with the Advanced Therapy Unit at King’s College London used MSCs to “prime” a monocyte cell therapy into one that was more favorably balanced towards anti-fibrosis and pro-angiogenesis. [64] Dr. Patel’s disease target— COVID-19 induced fibrotic interstitial lung disease—involves patients who exhibit severely restricted lung function from scarring after many months of recovery following SARS-CoV2 infection. Ex vivo manufacture of autologous monocytes for this trial could be greatly eased by enlisting an “off-the-shelf” bank of allogeneic MSCs from RoosterBio. Since this GMP bank of MSCs and paired media was qualified with Type II FDA Drug Master Files—and all necessary documentation, certification, and process development support—the journey from therapeutic concept to approval for a Phase I trial took just 8 months, instead of 12-18. In so doing, Patel and colleagues proved that promising, novel autologous cell therapies can effectively piggyback on an industrialized supply chain of allogeneic MSCs. In a larger sense, this is one prime exemplar of allogeneic cells employed to reduce the effort to launch an autologous cell therapy. Together with MSCs’ relative ease of genetic manipulation, the well-known synergies between MSCs and other immune modulatory cells will empower similar researchers’ creativity to tackle once inaccessible problems for those who wished to quickly enter early-Phase trials. Thus, a galaxy of new opportunity awaits younger T cell researchers who might otherwise find dismay when peering at an over-saturated oncology CAR-T space.
There are many more examples of ways T cells and MSCs can surely cooperate towards regenmed therapies and cures; this blog author didn’t list all, because it would fill a textbook. Nevertheless, when this 2030 edition textbook is written, it doesn’t stretch the imagination to predict it will be a top seller, with ample contributions from new investigators.
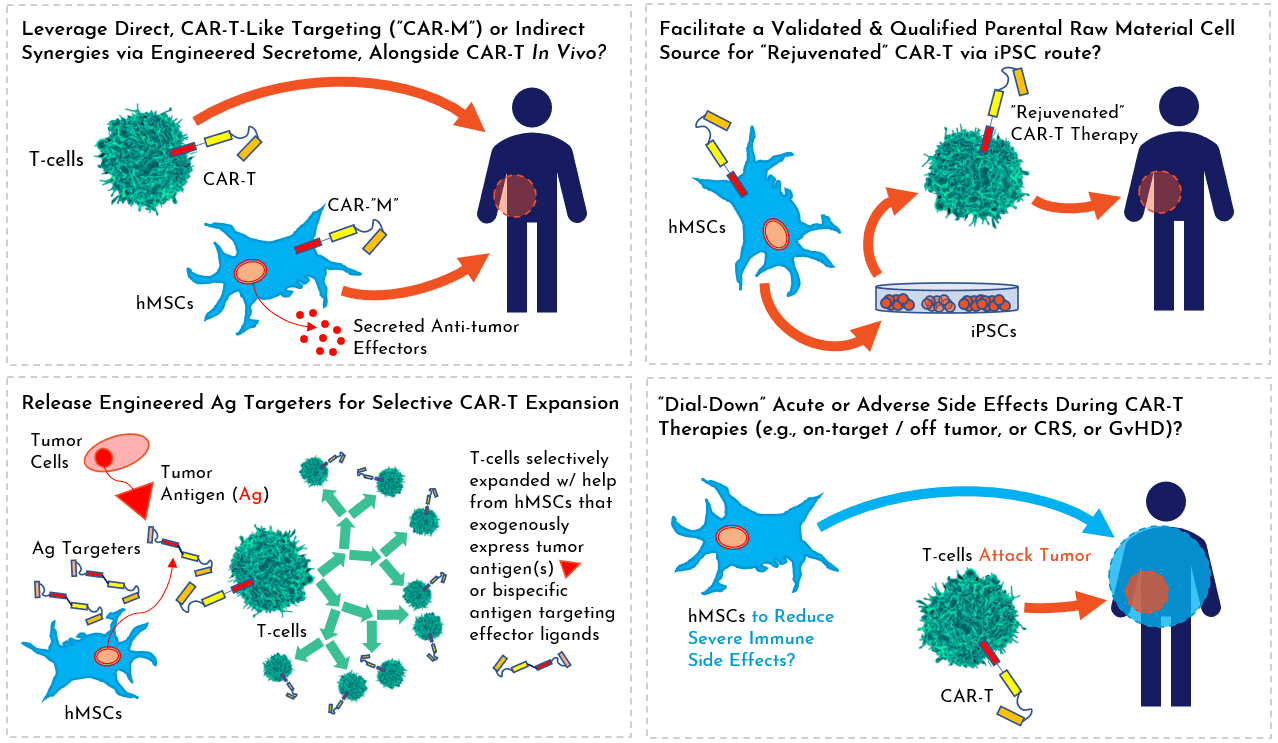 Illustration, above: Several hypothetical themes on ways MSCs could empower cell and gene therapies based on T cells.
Illustration, above: Several hypothetical themes on ways MSCs could empower cell and gene therapies based on T cells.
References:
- Heemskerk, B., et al., Adoptive cell therapy for patients with melanoma, using tumor-infiltrating lymphocytes genetically engineered to secrete interleukin-2. Hum Gene Ther, 2008. 19(5): p. 496-510. 10.1089/hum.2007.0171
- Park, T. S., S. A. Rosenberg, and R. A. Morgan, Treating cancer with genetically engineered T cells. Trends Biotechnol, 2011. 29(11): p. 550-7. 10.1016/j.tibtech.2011.04.009
- Amor, C., et al., Senolytic CAR T cells reverse senescence-associated pathologies. Nature, 2020. 583(7814): p. 127-132. 10.1038/s41586-020-2403-9
- Eyquem, J., et al., Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature, 2017. 543(7643): p. 113-117. 10.1038/nature21405
- Zhao, Z., et al., Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell, 2015. 28(4): p. 415-428. 10.1016/j.ccell.2015.09.004
- Globerson Levin, A., et al., CAR T cells: Building on the CD19 paradigm. Eur J Immunol, 2021. 51(9): p. 2151-2163. 10.1002/eji.202049064
- Braendstrup, P., B. L. Levine, and M. Ruella, The long road to the first FDA-approved gene therapy: chimeric antigen receptor T cells targeting CD19. Cytotherapy, 2020. 22(2): p. 57-69. 10.1016/j.jcyt.2019.12.004
- (FDA), US Food and Drug Administration. Cellular & Gene Therapy Products. FDA.gov 2021; Available from: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products.
- Rafiq, S., C. S. Hackett, and R. J. Brentjens, Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol, 2020. 17(3): p. 147-167. 10.1038/s41571-019-0297-y
- Boyiadzis, M. M., et al., Chimeric antigen receptor (CAR) T therapies for the treatment of hematologic malignancies: clinical perspective and significance. J Immunother Cancer, 2018. 6(1): p. 137. 10.1186/s40425-018-0460-5
- The Quest for Off-the-Shelf CAR T Cells. Cancer Discov, 2018. 8(7): p. 787-788. 10.1158/2159-8290.CD-ND2018-005
- Lim, W. A. and C. H. June, The Principles of Engineering Immune Cells to Treat Cancer. Cell, 2017. 168(4): p. 724-740. 10.1016/j.cell.2017.01.016allo
- MacLeod, D. T., et al., Integration of a CD19 CAR into the TCR Alpha Chain Locus Streamlines Production of Allogeneic Gene-Edited CAR T Cells. Mol Ther, 2017. 25(4): p. 949-961. 10.1016/j.ymthe.2017.02.005
- Lapteva, N., et al., T-Cell Receptor Stimulation Enhances the Expansion and Function of CD19 Chimeric Antigen Receptor-Expressing T Cells. Clin Cancer Res, 2019. 25(24): p. 7340-7350. 10.1158/1078-0432.CCR-18-3199
- Hurton, L. V., et al., Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proc Natl Acad Sci U S A, 2016. 113(48): p. E7788-E7797. 10.1073/pnas.1610544113
- Caplan, A. I. and D. Correa, The MSC: an injury drugstore. Cell Stem Cell, 2011. 9(1): p. 11-5. 10.1016/j.stem.2011.06.008
- Dimarino, A. M., A. I. Caplan, and T. L. Bonfield, Mesenchymal stem cells in tissue repair. Front Immunol, 2013. 4: p. 201. 10.3389/fimmu.2013.00201
- Gomez-Ferrer, M., et al., HIF-1alpha and Pro-Inflammatory Signaling Improves the Immunomodulatory Activity of MSC-Derived Extracellular Vesicles. Int J Mol Sci, 2021. 22(7). 10.3390/ijms22073416
- Podesta, M. A., G. Remuzzi, and F. Casiraghi, Mesenchymal Stromal Cells for Transplant Tolerance. Front Immunol, 2019. 10: p. 1287. 10.3389/fimmu.2019.01287
- Boberg, E., et al., Treatment of chronic GvHD with mesenchymal stromal cells induces durable responses: A phase II study. Stem Cells Transl Med, 2020. 9(10): p. 1190-1202. 10.1002/sctm.20-0099
- Lembong, J., et al., Bioreactor Parameters for Microcarrier-Based Human MSC Expansion under Xeno-Free Conditions in a Vertical-Wheel System. Bioengineering (Basel), 2020. 7(3). 10.3390/bioengineering7030073
- Jossen, V., et al., Manufacturing human mesenchymal stem cells at clinical scale: process and regulatory challenges. Appl Microbiol Biotechnol, 2018. 102(9): p. 3981-3994. 10.1007/s00253-018-8912-x
- Kirian, RD, et al., Scaling a xeno-free fed-batch microcarrier suspension bioreactor system from development to production scale for manufacturing XF hMSCs. Cytotherapy, 2019. 21(5): p. S71-S72.
- Olsen, T. R., et al., Peak MSC-Are We There Yet? Front Med (Lausanne), 2018. 5: p. 178. 10.3389/fmed.2018.00178
- Lembong, Josephine; Rowley, Jon. Building Effective Multi-Year Process Development Programs I: Estimating hMSC Lot Size Ranges for Clinical Manufacturing Through Commercial Demand. RoosterBio Blog 2021; Available from: https://www.roosterbio.com/blog/building-effective-multi-year-process-development-programs-i/
- Lembong, Josephine; Rowley, Jon. Building Effective Multi-Year Process Development Programs II: Evolution of Technology Platform Decisions Based on Lot Size. RoosterBio Blog 2021; Available from: https://www.roosterbio.com/blog/building-effective-multi-year-process-development-programs-ii-evolution-of-technology-platform-decisions-based-on-lot-size/
- Campbell, A., et al., Concise Review: Process Development Considerations for Cell Therapy. Stem Cells Transl Med, 2015. 4(10): p. 1155-63. 10.5966/sctm.2014-0294
- Robb, K. P., et al., Mesenchymal stromal cell therapy: progress in manufacturing and assessments of potency. Cytotherapy, 2019. 21(3): p. 289-306. 10.1016/j.jcyt.2018.10.014
- Witcher, Mark. Phase III Clinical Trials – Ever Wonder Why Some Products Unexpectedly Fail? Pharmaceutical Engineering 2019; Available from: https://ispe.org/pharmaceutical-engineering/ispeak/phase-iii-clinical-trials-ever-wonder-why-some-products-unexpectedly-fail.
- Rowley, J.; Carson, J. Adherent Cell Manufacturing’s Call to Action: Manufacturing Platform & Media Strategy Matters for Mesenchymal Stromal/Stem Cell Production Economics. RoosterBio Blog 2021; Available from: https://bit.ly/2UDvHFi.
- Mansouri, M., et al., Smart-watch-programmed green-light-operated percutaneous control of therapeutic transgenes. Nat Commun, 2021. 12(1): p. 3388. 10.1038/s41467-021-23572-4
- Kojima, R., L. Scheller, and M. Fussenegger, Nonimmune cells equipped with T-cell-receptor-like signaling for cancer cell ablation. Nat Chem Biol, 2018. 14(1): p. 42-49. 10.1038/nchembio.2498
- Balyasnikova, I. V., et al., Genetic modification of mesenchymal stem cells to express a single-chain antibody against EGFRvIII on the cell surface. J Tissue Eng Regen Med, 2010. 4(4): p. 247-58. 10.1002/term.228
- Golinelli, G., et al., Targeting GD2-positive glioblastoma by chimeric antigen receptor empowered mesenchymal progenitors. Cancer Gene Ther, 2020. 27(7-8): p. 558-570. 10.1038/s41417-018-0062-x
- Gerardo, H., et al., Soft culture substrates favor stem-like cellular phenotype and facilitate reprogramming of human mesenchymal stem/stromal cells (hMSCs) through mechanotransduction. Sci Rep, 2019. 9(1): p. 9086. 10.1038/s41598-019-45352-3
- Sharma, D. and F. Zhao, Updates on clinical trials evaluating the regenerative potential of allogenic mesenchymal stem cells in COVID-19. NPJ Regen Med, 2021. 6(1): p. 37. 10.1038/s41536-021-00147-x
- Kalluri, R. and V. S. LeBleu, The biology, function, and biomedical applications of exosomes. Science, 2020. 367(6478). 10.1126/science.aau6977
- Wiklander, O. P. B., et al., Advances in therapeutic applications of extracellular vesicles. Sci Transl Med, 2019. 11(492). 10.1126/scitranslmed.aav8521
- Camacho, V., et al., Bone marrow Tregs mediate stromal cell function and support hematopoiesis via IL-10. JCI Insight, 2020. 5(22). 10.1172/jci.insight.135681
- Yu, Q., et al., Advances in the treatment of graft-versus-host disease with immunomodulatory cells. Int Immunopharmacol, 2021. 92: p. 107349. 10.1016/j.intimp.2020.107349
- Safinia, N., et al., Successful expansion of functional and stable regulatory T cells for immunotherapy in liver transplantation. Oncotarget, 2016. 7(7): p. 7563-77. 10.18632/oncotarget.6927
- MacDonald, K. G., et al., Alloantigen-specific regulatory T cells generated with a chimeric antigen receptor. J Clin Invest, 2016. 126(4): p. 1413-24. 10.1172/JCI82771
- Biswas, M., et al., Gene Therapy With Regulatory T Cells: A Beneficial Alliance. Front Immunol, 2018. 9: p. 554. 10.3389/fimmu.2018.00554
- Sanchez-Fueyo, A., et al., Applicability, safety, and biological activity of regulatory T cell therapy in liver transplantation. Am J Transplant, 2020. 20(4): p. 1125-1136. 10.1111/ajt.15700
- Sawitzki, B., et al., Regulatory cell therapy in kidney transplantation (The ONE Study): a harmonised design and analysis of seven non-randomised, single-arm, phase 1/2A trials. Lancet, 2020. 395(10237): p. 1627-1639. 10.1016/S0140-6736(20)30167-7
- Harden, P. N., et al., Feasibility, long-term safety, and immune monitoring of regulatory T cell therapy in living donor kidney transplant recipients. Am J Transplant, 2021. 21(4): p. 1603-1611. 10.1111/ajt.16395
- Elinav, E., et al., Amelioration of colitis by genetically engineered murine regulatory T cells redirected by antigen-specific chimeric receptor. Gastroenterology, 2009. 136(5): p. 1721-31. 10.1053/j.gastro.2009.01.049
- Blat, D., et al., Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells. Mol Ther, 2014. 22(5): p. 1018-28. 10.1038/mt.2014.41
- Yoon, J., et al., FVIII-specific human chimeric antigen receptor T-regulatory cells suppress T- and B-cell responses to FVIII. Blood, 2017. 129(2): p. 238-245. 10.1182/blood-2016-07-727834
- Koristka, S., et al., Engrafting human regulatory T cells with a flexible modular chimeric antigen receptor technology. J Autoimmun, 2018. 90: p. 116-131. 10.1016/j.jaut.2018.02.006
- Fransson, M., et al., CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery. J Neuroinflammation, 2012. 9: p. 112. 10.1186/1742-2094-9-112
- Caplan, A. I., Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med, 2017. 6(6): p. 1445-1451. 10.1002/sctm.17-0051
- Lee, J. W., et al., Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med, 2013. 187(7): p. 751-60. 10.1164/rccm.201206-0990OC
- Salame, N., et al., UM171A-induced ROS promote antigen cross-presentation of immunogenic peptides by bone marrow-derived mesenchymal stromal cells. Stem Cell Res Ther, 2022. 13(1): p. 16. 10.1186/s13287-021-02693-z
- Szoor, A., et al., T Cell-Activating Mesenchymal Stem Cells as a Biotherapeutic for HCC. Mol Ther Oncolytics, 2017. 6: p. 69-79. 10.1016/j.omto.2017.07.002
- Hombach, A. A., et al., IL7-IL12 Engineered Mesenchymal Stem Cells (MSCs) Improve A CAR T Cell Attack Against Colorectal Cancer Cells. Cells, 2020. 9(4). 10.3390/cells9040873
- Zanetti, S. R., et al., Bone marrow MSC from pediatric patients with B-ALL highly immunosuppress T-cell responses but do not compromise CD19-CAR T-cell activity. J Immunother Cancer, 2020. 8(2). 10.1136/jitc-2020-001419
- van den Akker, F., et al., Suppression of T cells by mesenchymal and cardiac progenitor cells is partly mediated via extracellular vesicles. Heliyon, 2018. 4(6): p. e00642. 10.1016/j.heliyon.2018.e00642
- Wang, Q., et al., The allogeneic umbilical cord mesenchymal stem cells regulate the function of T helper 17 cells from patients with rheumatoid arthritis in an in vitro co-culture system. BMC Musculoskelet Disord, 2012. 13: p. 249. 10.1186/1471-2474-13-249
- Yang, H., et al., Umbilical cord-derived mesenchymal stem cells reversed the suppressive deficiency of T regulatory cells from peripheral blood of patients with multiple sclerosis in a co-culture – a preliminary study. Oncotarget, 2016. 7(45): p. 72537-72545. 10.18632/oncotarget.12345
- Cao, M., et al., Mesenchymal stem cells alleviate idiopathic pneumonia syndrome by modulating T cell function through CCR2-CCL2 axis. Stem Cell Res Ther, 2021. 12(1): p. 378. 10.1186/s13287-021-02459-7
- Caplan, H. W., et al., Combination therapy with Treg and mesenchymal stromal cells enhances potency and attenuation of inflammation after traumatic brain injury compared to monotherapy. Stem Cells, 2021. 39(3): p. 358-370. 10.1002/stem.3320
- Caplan, H. W., et al., Human-derived Treg and MSC combination therapy may augment immunosuppressive potency in vitro, but did not improve blood brain barrier integrity in an experimental rat traumatic brain injury model. PLoS One, 2021. 16(5): p. e0251601. 10.1371/journal.pone.0251601
- Alvaro, D., Patel, A. Accelerating the Development of a Novel Cell Therapy for COVID-19–Associated Lung Fibrosis. 2021; Available from: https://www.pharmasalmanac.com/articles/accelerating-the-development-of-a-novel-cell-therapy-for-covid-19-associated-lung-fibrosis.
