Amid the global search for treatments for COVID-19 related lung disorders, MSCs emerged as one of the promising solutions to treat Acute Respiratory Distress Syndrome (ARDS) [1, 2]. (See our previous blog posts on this topic and our scalable hMSC manufacturing approach in response to COVID-19). Due to the similar therapeutic properties of exosomes and extracellular vesicles (EVs) and their parent cells, the role of MSC-EVs in treatment of lung injuries is being investigated [3, 4, 5]. Based on our previous estimate, treating 10,000 patients at a dose of 300M cells/patient requires a total of 6.4 trillion cells to be manufactured*. Even in the best-case scenario where a 500L bioreactor process for MSCs is already developed, this will take 22 production runs per year – which is still a significant burden for any manufacturing site.
* It is more challenging to estimate what this scale looks like for MSC-EVs due to limited clinical data, but we’ll discuss more of that a little later in this post.
The key takeaway is that scalable bioprocess is critical to rapidly deploy MSCs or MSC-EVs as a widespread treatment. This sort of quantitative exercise is important to plan your therapeutic product development program and build it accordingly to meet the calculated lot size and production schedule. Diligent process development and optimization that is scalable can help smooth the path to the clinic.
There is need for a standardized way to evaluate and compare upstream and downstream processes for MSC and MSC-EV production. Although this blog is dedicated to upstream aspects of effective scale-up for EV production, others have written skillfully on crucial downstream processing considerations, e.g. purification [6, 7] and storage [8]. To address cell culture & EV productivity, we propose a few key metrics, as well as a few examples of manufacturing considerations that influence these metrics. These manufacturing decisions can greatly affect the product yield and development timeline, thus critically impacting the deployment of these therapies for the current pandemic.
Productivity metrics for MSC and MSC-EV-based therapies
Whether MSCs or MSC-EVs is the final product for treatment of ARDS, the goal of any therapeutic development is to maximize the number of doses per production lot while maintaining product CQAs / efficacy. As large-scale manufacturing of MSCs and MSC-EVs are being developed for use in therapies, productivity metrics need to be defined to measure of the efficiency of MSC or MSC-EV generation. Understanding these metrics will help inform the critical decision of reagent selection, production platform, and downstream processing.
The monoclonal antibody (MAb) field had defined and used a productivity metric: grams per liter. More recently, the cell therapy manufacturing field defined a similar metric as a measure of media productivity [9], as quantified by millions of cells produced per liter of media consumed [10]. This metric outlines the technological and economic aspects of a bioprocess run, as media is a major cost driver in production. One way to maximize this productivity is through an efficient fed-batch media strategy [11].
When defining EV productivity, this measure of efficiency of EV generation can be captured in various parameters: (1) the biological productivity (EVs per cell), (2) process efficiency (EVs per day), and (3) cost efficiency (EVs per liter of media).
- The biological productivity metric (EVs per cell) outlines the biological aspects of EV production, and is intrinsic to the cell type, as shown by the various level of EVs secreted by various cell types [12]. MSCs, while itself is a promising therapeutic for ARDS, are also one of the most efficient primary cell source of EVs [12]. In addition, culture conditions have also been shown to alter the number of EVs produced per cell, e.g. 3D microcarrier-based hMSC culture in a suspension bioreactor produced twice the number of EVs per cell compared to the traditional 2D flask culture [13].
- The process efficiency (EVs per day) incorporates time as a consideration when developing a manufacturing process. This is critical since minimizing GMP manufacturing time directly translates to meaningful decreases in COGS as related to reduced labor, GMP facility costs, and the associated overhead costs. The process efficiency metric allows the comparison of various culture and manufacturing processes for EV generation. To optimize this process efficiency, process parameter optimization (cell age, seeding density, expansion duration, and EV collection duration) is often performed in small-scale cultures [14]. In addition, some media components / additives have been shown to increase the rate of EV production, such as an EV Boost™ [15] and low concentrations of ethanol [16], therefore increasing the process efficiency.
The cost productivity metric, EVs per ml media, reflects the volume of media required to generate a fixed EV yield, which directly correlates to cost. We have seen that hMSC cultures in bioreactors produce higher EV concentration per ml media compared to 2D flask cultures [13, 17]. Bioreactors for EV production can operate with different media paradigms (i.e. perfusion, microcarrier suspension, and media exchanges to allow multiple collections), which affect media costs. While increasing the frequency of EV collection can increase total EV yield [14], the use of a higher media volume decreases this cost productivity metric and allows for cost-driven quantitative data to evaluate the utility of multiple collections. The cost productivity metric can also be applied to analyze EV downstream processing platforms. For example, quantifying this metric for pre-and post-processing samples using ultracentrifugation and tangential flow filtration (TFF) allows analysis of the system efficiency in terms of the concentration and volume reduction [18].
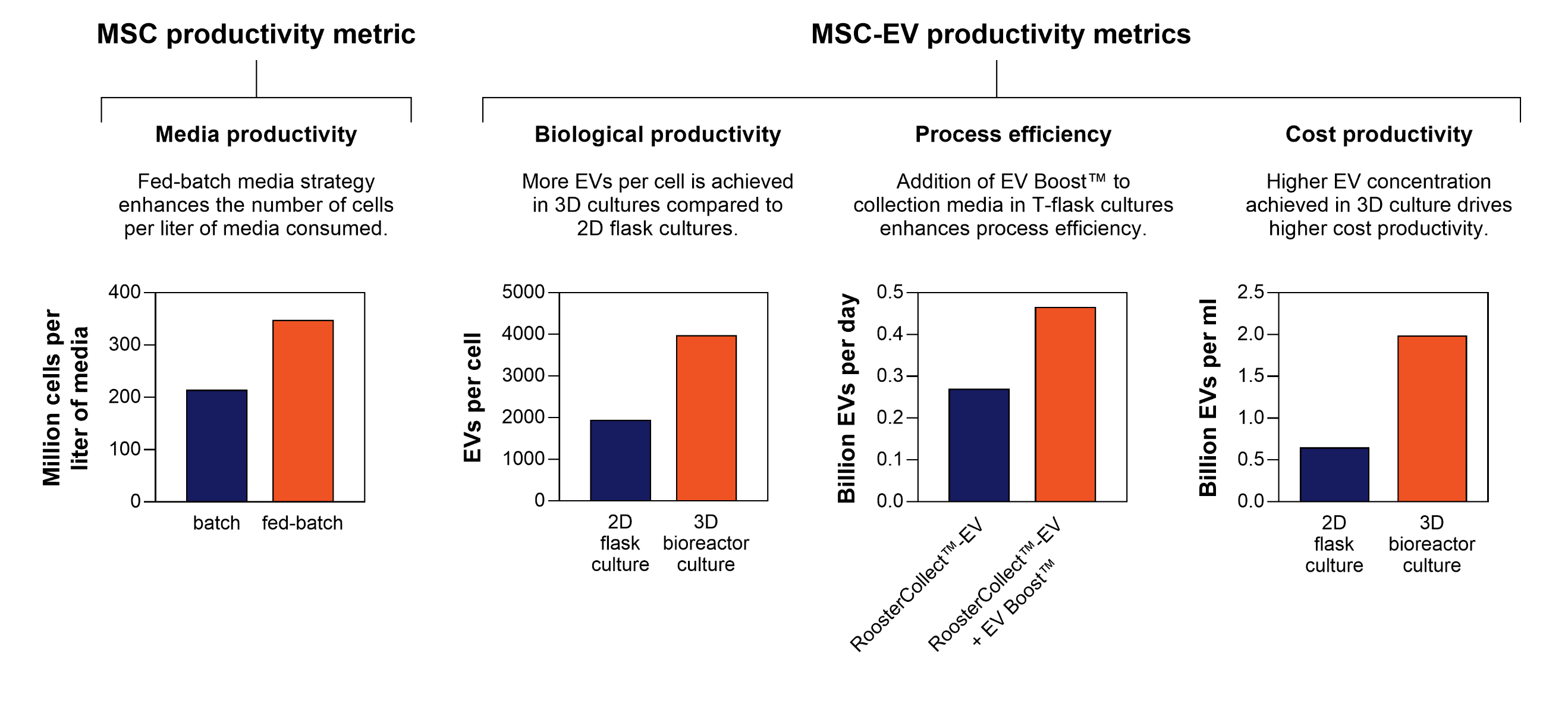 Figure 1. MSC and MSC-EV productivity metrics achieved using RoosterBio’s cell and media systems. A few manufacturing aspects, such as media paradigm, production platform, and media reagent selection, heavily influence these metrics.
Figure 1. MSC and MSC-EV productivity metrics achieved using RoosterBio’s cell and media systems. A few manufacturing aspects, such as media paradigm, production platform, and media reagent selection, heavily influence these metrics.
“Give more, get more” – Higher productivity via manufacturing scale-up
Since clinical studies of MSC-EVs for treatment of ARDS are still in their infancy, estimating the target manufacturing requirements for MSC-EVs is challenging. Different models are being used in quantifying the dose of EVs [4], which makes it difficult to make direct comparisons between studies. Starting with the MSC-EV doses that have been reported in pre-clinical models for lung injuries [19, 20, 21, 22], a relevant dose of EVs for a human can be estimated to be released from anywhere between 50M cells and 50B cells. The EVs released from the estimated 300 million hMSCs needed for ARDS treatments falls within this range, and is therefore, to first approximation, likely a relevant dose for this indication.
We can perform a similar exercise to estimate the number of required MSC-EV manufacturing lots to meet the clinical demand. Assuming a conservative biological productivity of ~1500 EV / cell yield [14], an average dose can be estimated to be 4.5×1011 EVs per patient. The total demand of EVs to treat 10,000 patients is therefore calculated to be 9.6×1015 EVs (from ~6.4 Trillion cells). Let’s incorporate our biological productivities using our cell & media systems (From Figure 1: ~2000 EVs/cell in 2D flask culture, and ~4000 EVs/cell in 3D systems) into the various production platforms and scales.
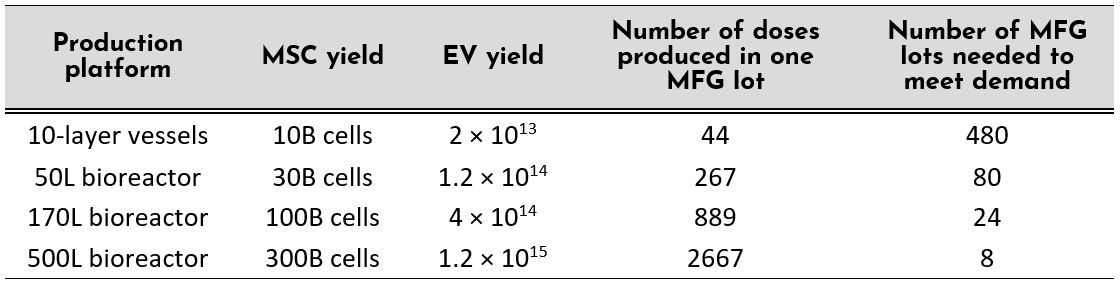
- A switch from 2D to 3D production platform can produce >6x the number of EV doses. Availability of bioreactor platforms up to 2000L scale can further increase the number of doses produced per run, decreasing the number of productions down to twice a year to meet the current demand.
- If clinical-grade reagents designed to enhance process efficiency are implemented into the therapeutic manufacturing, this will relax the production schedule to meet the clinical demand; or more production lots can be done per year if operating at the same capacity.
- Reduction in the number of production lots due to these productivity enhancements have positive implications further downstream of manufacturing: decreased frequency of release testing and maintenance of lot stability, as well as its associated costs.
Quantifying these productivity metrics can aid product developers in making data-driven decisions on their process design that can generate cells and EVs efficiently. Taking such a bioprocess approach towards MSC and MSC-EV production allows companies to achieve clinically relevant lot sizes of MSC and MSC-EV products for treatment of COVID19-related lung disorders.
RoosterBio offers a complete system for EV production that can readily be implemented in late stage product development and cGMP manufacturing. It is the only system designed with hMSC-EV product developers in mind. Our CliniControl™ product portfolio offers high volume, affordable, and well-characterized hMSCs paired with highly engineered media in order to advance your EV product into the clinic. With the new addition of RoosterCollect™-EV-CC to RoosterBio’s CliniControl™ product solutions, EV product developers now have a path to a productive, scalable and industrialized cGMP hMSC-EV production. Contact us now to learn more about how this hMSC-EV System may benefit your research and/or product development!
References
- Matthay, M.A., Therapeutic Potential of Mesenhttps://www.roosterbio.com/products/roostercollect-ev-cc-m02001/chymal Stromal Cells for Acute Respiratory Distress Syndrome. Ann Am Thorac Soc, 2015. 12: p. S54-S57.
- Matthay, M., et al., Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med, 2019. 7(2): p. 154-162.
- Liu, A., et al., Therapeutic potential of mesenchymal stem/stromal cell-derived secretome and vesicles for lung injury and disease. Expert Opin Biol Ther, 2020. 20(2): p. 125-140.
- Worthington, E.N. and J.S. Hagood, Therapeutic Use of Extracellular Vesicles for Acute and Chronic Lung Disease. Int J Mol Sci, 2020. 21(7).
- Khoury, M., et al., Cell-Based Therapies for COVID-19: Proper Clinical Investigations are Essential. Cytotherapy, 2020.
- Lee, Y.X.F., et al., Considerations and Implications in the Purification of Extracellular Vesicles – A Cautionary Tale. Front Neurosci, 2019. 13: p. 1067.
- Mol, E.A., et al., Higher functionality of extracellular vesicles isolated using size-exclusion chromatography compared to ultracentrifugation. Nanomedicine, 2017. 13(6): p. 2061-2065.
- Jeyaram, A. and S.M. Jay, Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J, 2017. 20(1): p. 1.
- Rowley, J.A. and S.A. Montgomery, The Need for Adherent Cell Manufacturing: Production Platform and Media Strategies Drive Cell Production Economics. BioProcess International, 2018. 16(11-12): p. 34-49.
- The Glossary for Cell & Gene Therapy and Regenerative Medicine. Regenerative Medicine, 2018. 13(8S).
- Kirian, R., et al., Scaling a xeno-free fed-batch microcarrier suspension bioreactor system from development to production scale for manufacturing XF hMSCs. Cytotherapy, 2019. 21(5): p. S71-S72.
- Yeo, R.W., et al., Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev, 2013. 65(3): p. 336-41.
- Adlerz, K., et al., Comparison of MSC-EVs Manufactured in 2D versus Scalable 3D Bioreactor Systems. Cytotherapy, 2019. 21(5): p. S58.
- Patel, D.B., et al., Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell-derived extracellular vesicles. Bioeng Transl Med, 2017. 2(2): p. 170-179.
- Adlerz, K., et al., Increasing Yield of MSC-EVs in Scalable Xeno-free Manufacturing. Cytotherapy, 2019. 21(5): p. S58.
- Lamichhane, T.N., et al., Ethanol Induces Enhanced Vascularization Bioactivity of Endothelial Cell-Derived Extracellular Vesicles via Regulation of MicroRNAs and Long Non-Coding RNAs. Sci Rep, 2017. 7(1): p. 13794.
- Watson, D.C., et al., Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials, 2016. 105: p. 195-205.
- Busatto, S., et al., Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells, 2018. 7(12).
- Khatri, M., L.A. Richardson, and T. Meulia, Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther, 2018. 9(1): p. 17.
- Phinney, D.G. and M.F. Pittenger, Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells, 2017. 35(4): p. 851-858.
- Morrison, T.J., et al., Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am J Respir Crit Care Med, 2017. 196(10): p. 1275-1286.
- Zhu, Y.G., et al., Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells, 2014. 32(1): p. 116-25.
