Human Mesenchymal Stem Cells or Mesenchymal Stromal Cells, the “workhorse” of Regenerative Medicine (RegenMed), are a critical starting material in a growing variety of established and emerging RegenMed products. [1],[2] hMSC’s unique balance of functional bioactive attributes, expansion potential, and established safety profile have resulted in steady increases in both scientific publications [3] and clinical trials (CT) [4] over the past 20+ years.
RoosterBio Products Expanding Use in High Impact Publications
We are encouraged that over the past 6 years, RoosterBio’s core technologies and high quality hMSCs paired with highly productive bioprocess media, have been used in a steadily increasing number of peer-reviewed publications (Fig. 1), which we affectionately call RoosterPublications. This past year was no exception, as 2019 featured a total of 47 articles utilizing RoosterBio products; a 34% increase over 2018. A sortable list of these publications can now be found in our new RoosterBio Knowledge Center. As RoosterBio continues its focus on affordable, innovative products to the greater research community, we expect that this increase will continue in the coming years.
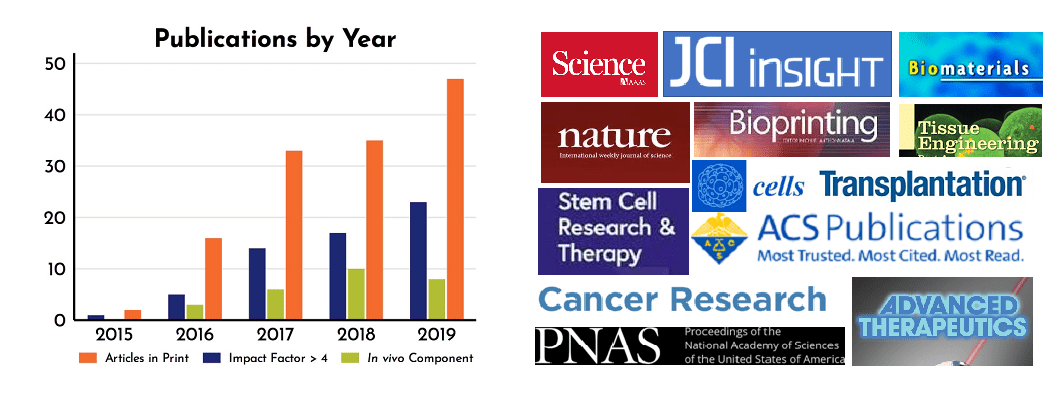
Figure 1: Yearly increase in publications containing RoosterBio products (left) including an increase in high impact journals (right).
In addition to total publications, we track articles using journal Impact Factor (IF), an index of citations per publication, often used as a metric for a journal’s importance in its field. [5] Like total papers, the number of articles featuring our products in journals with an IF >4 are increasing year over year (Figure 1). Recent high impact journals where RoosterPublications are found include ACS Biomaterials Science & Engineering, Stem Cell Research & Therapy, Bioprinting, ACS Biomacromolecules, Journal of Clinical Investigation Insight, Cancer Research, PNAS, Biomaterials, and Science (Figure 1).
RoosterPublications Are Expanding Their Impact on the RegenMed Space
The breadth of the research areas in RoosterPublications are diverse, and covers an expansive part of the RegenMed space. To categorize and to access the impact of the RoosterPublications, we divided the RegenMed space into 7 Technical Segments:
- Cell Therapy: hMSCs are used as the therapeutic agent
- Exosomes, EVs, & Secretome: hMSCs are used to produce EVs and/or proteins
- Genetic Engineering: gene modified hMSCs for added potency, homing, etc.
- Med Tech/Device: hMSCs used in combination with a device or biomaterial
- Tissue & Organ Engineering: hMSCs as building block for engineered tissues or organs
- Bioprinting: hMSCs used as part of ink (closely related to Tissue & Organ Engineering)
- BioProcess Tool Developers: hMSCs used in developing bioreactors, cryopreservation, or separation tools and reagents
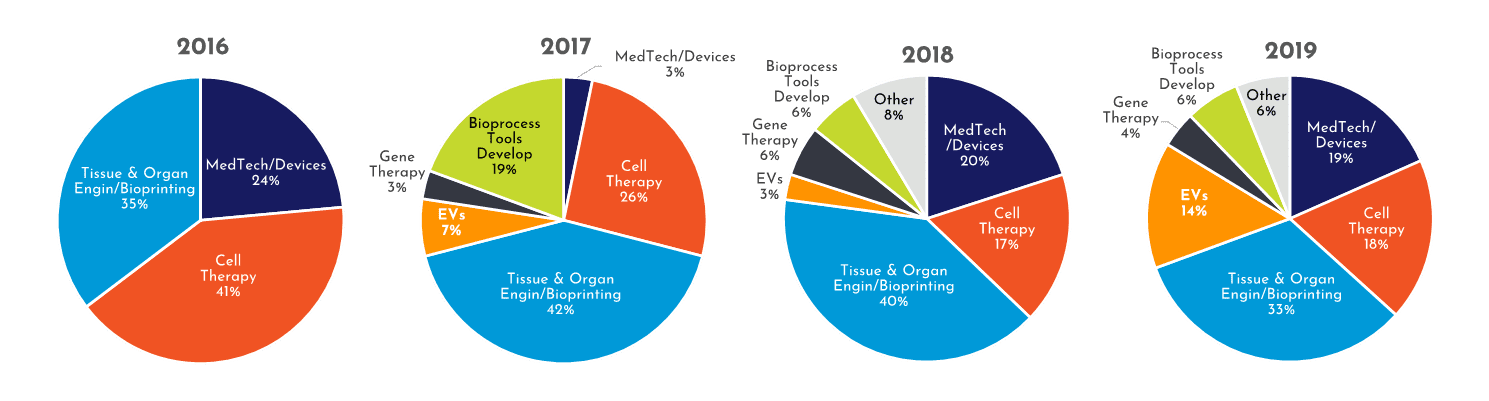
Figure 2: Growing diversity of applications using RoosterBio product within publications. (note: Tissue & Organ Engineering combined with Bioprinting due to overlap)
RoosterPublications have been present in all 7 Tech Segments since 2017 (Figure 2). Yet, as shown in Figure 2, the diversity of RoosterPublications has increased over time. For example, the number of RoosterPublications in Tech Segments outside of Cell Therapy, Tissue and Organ Engineering, and Bioprinting has increased from 32% to 49% (Figure 3). As RoosterPublications accelerate in their impact, we expect this trend to continue with larger increases in the “hot”, developing Tech Segments of Gene Therapy and Extracellular Vesicles.
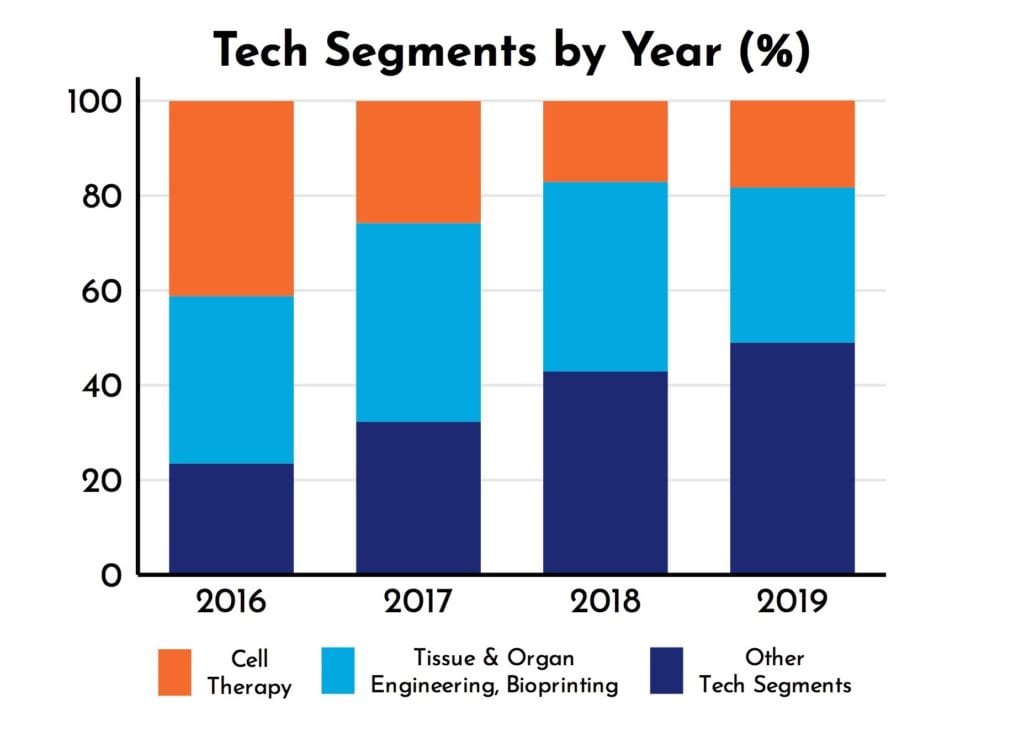
Figure 3: Emerging Tech Segment increase in publication prevalence.
Highlighted 2019 RoosterPublications in Each Tech Segment
It is refreshing to see the innovative work happening across the RegenMed space, and we’re particularly honored to highlight a few of these representative studies (below):
-
Cell Therapy
Programmable microencapsulation for enhanced mesenchymal stem cell persistence and immunomodulation.
Mao AS, Özkale B, Shah NJ, Vining KH, Descombes T, Zhang L, Tringides CM, Wong SW, Shin JW, Scadden DT, Weitz DA, Mooney DJ. PNAS, 2019.
-
Exosomes, EVs, & Conditioned Media
Hogan SE, Rodriguez Salazar MP, Cheadle J, Glenn R, Medrano C, Petersen TH, Ilagan RM. Am J Physiol Lung Cell Mol Physiol, 2019.
Mansouri N, Willis GR, Fernandez-Gonzalez A, Reis M, Nassiri S, Mitsialis SA, Kourembanas S.
JCI Insight, 2019.
-
Genetic Engineering
Development of MicroRNA-146a-Enriched Stem Cell Secretome for Wound-Healing Applications.
Waters R, Subham S, Pacelli S, Modaresi S, Chakravarti AR, Paul A. Mol Pharm, 2019.
-
MedTech / Devices
Heparin/Collagen Coatings Improve Human Mesenchymal Stromal Cell Response to Interferon Gamma
Castilla-Casadiego DA, García JR, García AJ, and Almodovar J. ACS Biomaterials Science & Engineering, 2019.
-
Tissue & Organ Engineering
Three-Dimensional Printed Polylactic Acid Scaffolds Promote Bone-like Matrix Deposition in Vitro.
Fairag R, Rosenzweig DH, Ramirez-Garcialuna JL, Weber MH, Haglund L. ACS Appl Mater Interfaces, 2019.
-
Bioprinting
Piard C, Jeyaram A, Liu Y, Caccamese J, Jay SM, Chen Y, Fisher J. Biomaterials, 2019.
-
BioProcess Tools Developers
Scaled-Up Inertial Microfluidics: Retention System for Microcarrier-Based Suspension Cultures.
Moloudi R, Oh S, Yang C, Teo KL, Lam AT, Ebrahimi Warkiani M, Win Naing M. Biotechnol J, 2019.
Across the life sciences, the timeframe from project initiation to first publication is long; with a current average of 3 to 4 years. [6] RoosterBio’s high volume hMSCs and bioprocess media systems are uniquely engineered to allow researcher to spend less time in preparative cell culture and more time gathering data from experiments. Ultimately, our goal is to enable researchers to utilize products that accelerate their timelines via well-characterized, consistent raw materials — optimized to simplify work flows, reduce costs, and shorten hands-on time for research, development, or clinical translation of the world’s next-gen cellular therapies.
Additional References
- Pittenger, M. F., et al., Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med, 2019. 4: p. 22. 10.1038/s41536-019-0083-6
- Olsen, T. R., et al., Peak MSC-Are We There Yet? Front Med (Lausanne), 2018. 5: p. 178. 10.3389/fmed.2018.00178
- NCBI. PubMed Query, “Mesenchymal Stem” OR “Mesenchymal Stromal”. National Library of Medicine 1975-2020; Available from: https://bit.ly/3hsGmd3.
- NCBI. Clinicaltrials.gov query, “mesenchymal stem” OR “mesenchymal stromal” AND AREA[StudyType] EXPAND[Term] COVER[FullMatch] “Interventional”. National Library of Medicine 2020; Available from: https://bit.ly/2UOVlUG.
- McKiernan, E. C., et al., Use of the Journal Impact Factor in academic review, promotion, and tenure evaluations. Elife, 2019. 8. 10.7554/eLife.47338
- Vale, R. D., Accelerating scientific publication in biology. Proc Natl Acad Sci U S A, 2015. 112(44): p. 13439-46. 10.1073/pnas.1511912112
