New Data Highlight Exciting Uses for Future Immunotherapy Studies
Genetic engineering is a cornerstone of the cell therapy field, upon which very successful and effective treatments like Kymriah and Luxturna are built. We need not look further than the examples of chimeric antigen receptor (CAR) T cells [1, 2] and genome-edited “neoTCR” cells. [3, 4] In these groundbreaking treatments, patient T cells are isolated and “reprogrammed” to express CARs or personalized T cell receptors (TCRs) that will recognize and bind to specific antigens, such as surface markers or HLA peptides that are highly selective for cancerous cells. Upon binding their targets, millions of T cells can massively and swiftly eliminate billions of cancer cells in a single patient. To reprogram T cells, lentivirus is often used as the shuttle to introduce the CAR or TCR DNA into the T cell genome. While lentivirus does its job nicely, there is always room for improvement.
We at RoosterBio are now discovering that our RoosterGEM™ product is akin to a Swiss Army Knife. [5, 6, 7] This jack of all trades has been shown to enhance lentiviral and AAV transduction of adherent primary cells like mesenchymal stromal/stem cells (MSCs), and even dramatically boost transient transfection of MSCs with mRNA. Furthermore, RoosterGEM does not have any licensing “strings” attached. It’s ready to purchase and use at point of process, with a GMP formulation soon to hit the scene.
With all the benefits of RoosterGEM in mind, we turned our sights to the genetic engineering of clinically relevant immune cells. Despite their great utility, primary cells such as T cells can be challenging to transduce with high efficiency. [8] To partially overcome this challenge, T cells extracted via leukapheresis can be subject to a complex manufacturing process involving activation cytokines, and higher multiplicities of infection (MOI) could also be used. However, this is not inexpensive and moreover, smaller MOIs to yield <5 copies per genome are recommended by the FDA to minimize risk of insertional mutagenesis. [9]
To our surprise, we recently determined that RoosterGEM enhances the transduction efficiency of T cells, reducing the amount of lentivirus required for a given process. We first honed in on some basic conditions to enhance lentiviral transduction of primary human T cells with RoosterGEM (Figure 1, below). When we applied RoosterGEM in transductions at the low MOI of 2, our qualitative view of GFP+ expression under fluorescent microscopy showed us dramatically enhanced numbers of transgene-positive cells.
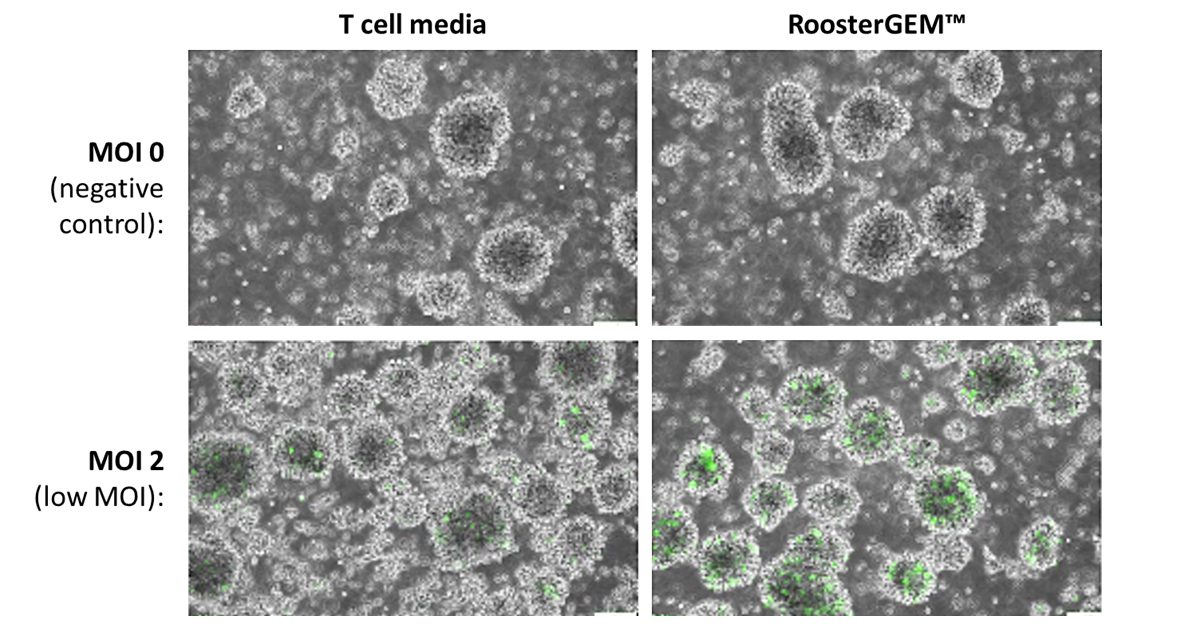
Figure 1 (above). Representative fluorescence microscopy images of T cells transduced with GFP-expression lentivectors at low MOI of 2 (lower panels), compared with the mock negative control (MOI=0; upper panels). Left panels, T cells transduced in culture media only. Right panels, transduction of T cells with RoosterGEM™.
To further probe the scope of RoosterGEM benefit, we transduced activated T cells using the GFP lentivirus at MOIs of 0, 2, 5, 10, or 20 (Figure 2, below). Cells were transduced in T cell expansion medium, T cell expansion medium supplemented with a commercially available agent, or T cell expansion medium supplemented with a special formulation of 50x concentrated RoosterGEM to yield a final concentration of 1x RoosterGEM. High-density cells were incubated with lentivirus overnight before being centrifuged and resuspended at a low density. Use of RoosterGEM did not significantly alter T cell viability, indicating its compatibility with sensitive immune cells. Upon running flow cytometry, it was observed that RoosterGEM increased the GFP-positive population and increased the average mean fluorescent intensity of GFP-positive cells compared to control. In other words, RoosterGEM enhanced T cell transduction efficiency and enhanced T cell productivity.
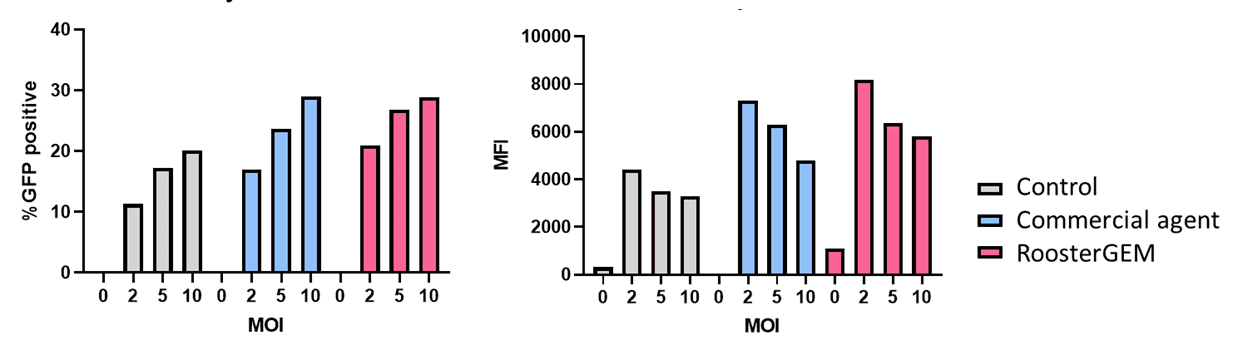
Figure 2 (above). Flow cytometry data of T cells transduced with GFP-expression lentivectors at dose range of MOIs, from 0 to 10, harvested on Day 3 post-transduction. Left graph, percent cells expressing GFP. Right graph, mean fluorescence intensity (MFI) of transduced cells. Grey bars, control media. Blue bars, comparative data obtained through use of a putative commercial lentiviral transduction enhancer. Pink bars, RoosterGEM data.
Through our own efforts—and through the work of our partners, customers and collaborators—we’re quickly discovering that the mechanism behind RoosterGEM treatment is widely applicable to diverse cell types and diverse gene transfer modalities. We’re also working out optimal conditions to use this multi-faceted “GEM” for GMP grade production of cell volumes equating to human-sized doses, [6] and will soon be updating you on those exciting efforts.
Do you think that you might have a need for enhanced transduction and/or transfection efficiency for your immunotherapy bioprocess? We’re ready to assist with suggestions regarding how to implement a working protocol based on our ongoing findings from primary T cells, and will be sure to keep you posted on new developments and optimizations.
References
- Gross, G., et al., Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. Transplant Proc, 1989. 21(1 Pt 1): p. 127-30.
- Grupp, S. A., et al., Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med, 2013. 368(16): p. 1509-1518. 10.1056/NEJMoa1215134
- Foy, S. P., et al., Non-viral precision T cell receptor replacement for personalized cell therapy. Nature, 2022. 10.1038/s41586-022-05531-1
- Ledford, H., CRISPR cancer trial success paves the way for personalized treatments. Nature, 2022. 611(7936): p. 433-434. 10.1038/d41586-022-03676-7
- Willstaedt, Terri. Genetically Engineered Mesenchymal Stromal Cells (MSCs): A Promising Tool for the Successful Delivery of Therapeutic Genes. 2021; Available from: https://www.roosterbio.com/blog/genetically-engineered-mesenchymal-stromal-cells-a-promising-tool-for-the-successful-delivery-of-therapeutic-genes/.
- Development of an Optimized Lentiviral Transduction Medium and Process to Manufacture Genetically Modified MSC Working Cell Banks (Poster). Available from: https://www.roosterbio.com/wp-content/uploads/2021/05/202105-ISCT_Terri_Willstaedt_121.pdf.
- RoosterBio. RoosterGEM™ Expands into New Fields of “Cell-Grow-Culture” for mRNA Transfection. 2022; Available from: https://www.roosterbio.com/blog/roostergem-expands-into-new-fields-of-cell-grow-culture-for-mrna-transfection/.
- Lo Presti, V., et al., Efficient lentiviral transduction method to gene modify cord blood CD8(+) T cells for cancer therapy applications. Mol Ther Methods Clin Dev, 2021. 21: p. 357-368. 10.1016/j.omtm.2021.03.015
- Zhao, Y., H. Stepto, and C. K. Schneider, Development of the First World Health Organization Lentiviral Vector Standard: Toward the Production Control and Standardization of Lentivirus-Based Gene Therapy Products. Hum Gene Ther Methods, 2017. 28(4): p. 205-214. 10.1089/hgtb.2017.078
