“…After many years have slipped by, the leaders of the Greeks,
opposed by the Fates, and damaged by the war,
build a horse of mountainous size…
They secretly hide a picked body of men, chosen by lot,
there, in the dark body, filling the belly and the huge
cavernous insides…” 1
What cannot be accomplished with endless brute force is sometimes possible with a little finesse. Although the “Trojan Horse” analogy’s been applied countless times since ~1200 BCE, it’s especially apt for that tricky challenge of gene transfer into primary cells. Here, we report how the quest to build a reliable “Trojan Horse” for ex vivo gene therapy could be assisted by a “shiny” new tool, perhaps coming from a Rooster bearing gifts?
To discuss these developments, in 2023 RoosterBio hosted a well-attended webinar, Solving Challenges in Viral and Non-Viral Cell Engineering for Advanced Therapies, where we relayed our latest findings on why it might be productive to “Troy” 🙃 something new:
|
Why?
Jon Carson began the webinar by noting how emerging synthetic biology tech is opening a whole new frontier for medicine. That is, while cell phones are programmable with software “apps,” cell therapies can be programmable with engineered DNA sequences, the software of life. The increase in computational power with Moore’s law can be paralleled by trends in improved DNA reading, writing, and editing to fight unmet medical needs with unprecedented control and potency.
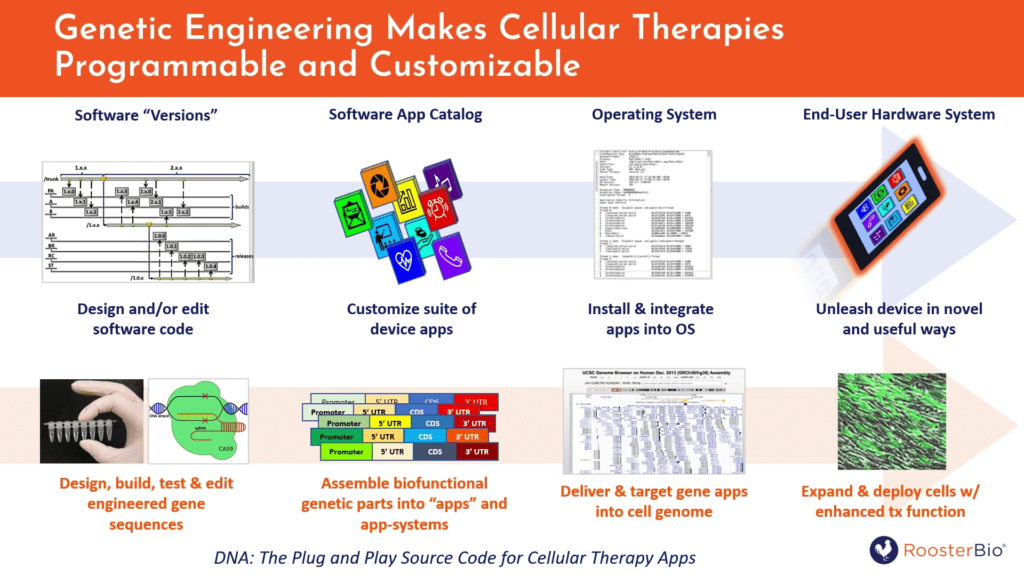
Figure 1. DNA imagined as a “plug and play” source code for loading of “apps” into human ex vivo modified cells, for the re-design of existing, natural biological system for useful purposes.
Dr. Carson also explained why “synbio” could make a difference beyond the current state of the art. First, deterministic genetic modification could enhance the cellular material’s viability span during ex vivo manufacture and/or in vivo treatment. Second, genetic tweaks may lead to improved manufacturability of the cell-drug, e.g., by single cell reporting on a quality metric such as the presence of the specific gene edit. Third, expressed gene products vastly increase the potency of the cell’s innate therapeutic activity; this happens by optimization of the cell’s targeting, homing, or secretome profile. Finally, genetic controls and effectors boost what was once a heterogeneous group of cells into a standardized delivery system for a gene therapy; the dominant phenotype contributed by the genetic “app system” facilitates a clear definition of monitorable critical quality attributes (CQAs) and process parameters (CPPs) that can uniformly pass muster during bioproduction scale-up.
These advantages are not merely abstract. Carson noted a prime example of what happens when a gene therapy meets cell therapy in the history of CAR-T (Chimeric Antigen Receptor) therapy. Once, T cells had been often considered in experimental therapies for non-gene-modified transfers, such as with neoantigen-specific clones expanded ex vivo and then adoptively transferred. Although genetic modification with CAR-T configurations had been empirically explored for decades, it took a watershed event via Dr. Carl June’s group’s 2013 widely-cited publication in 2013 2 to grab the world’s attention. Thereafter, search query language reflective of non-gene-modified T cells diminished and gave way to a flood of CAR-T related publications and trials that continues to this day. In fact, the CAR-T “revolution” kickstarted a trend to fill the FDA’s roster of approved Advanced Therapies with new products that were both gene therapies and cell therapies. It would almost seem as if gene therapies need cells—and cells need gene therapies—to cross the finish line!
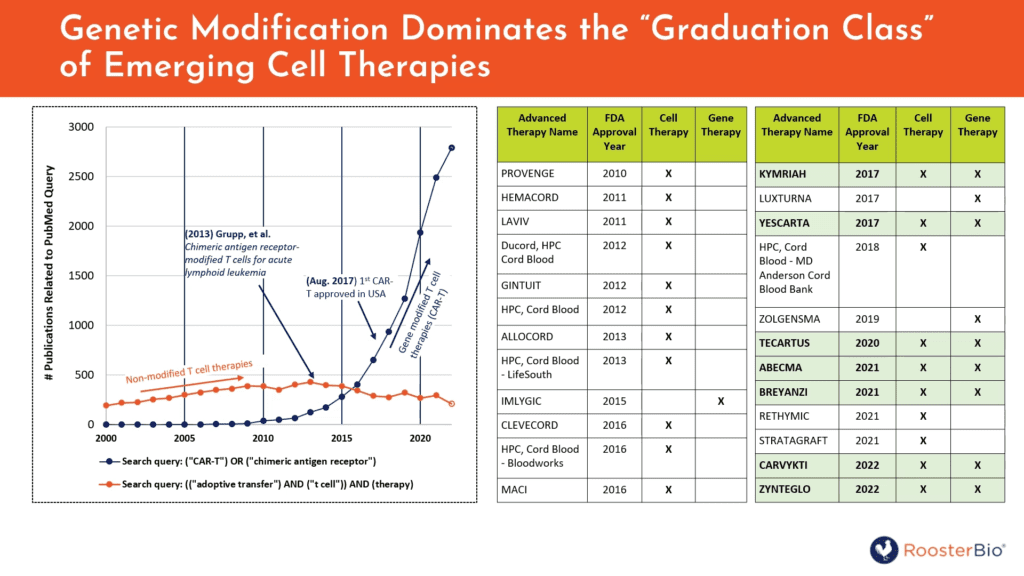
Figure 2. Left, targeted queries to PubMed indicate how research involving genetic modification of T cells (“CAR-T”) seems to have displaced some query hits implying non-genetically modified T-cell therapies (“adoptive transfer”). Right, the list of FDA-approved Advanced Therapies includes an increasing fraction of products that are both cell therapies and gene therapies, i.e., cells employed as targetable carriers for gene-controlled bioactivity against disease.
Carson next described another trend in bioscience research that is only now approaching clinical translation in human trials: exosomes and/or extracellular vesicles (EVs). Surprisingly, EVs/exosome publications now earn more query hits in PubMED than those related to liposomes or monoclonal antibodies (mAbs)—the latter now being the cornerstone technology of a ~$180 billion/year industry sector. Like mAbs, exosomes/EVs can be used as a targeting system for a molecular therapeutic activity (e.g., a mAb’s ADCC), undergirded by affinity matured, recombinant gene sequences. Like liposomes, exosomes/EVs can encapsulate therapeutic bioactivities and protect them from degradation in serum. But unlike CHO cells, the ideal producer cells for many exosomes/EVs might well turn out to be primary cells, not necessarily “classic” cell lines.
Herein lies a problem that begs for a solution! Since T cells, HSCs, NK cells, NSCs, hMSCs, and other cell types employed for therapeutic gene engineering are primary cells, they’re often much harder to transfect or transduce than established cell lines. That is, the “Trojan Horse” gene transfer “gift” is somehow more welcome in cells immortalized and/or transformed with oncogenes. Yet when delicate primary cells are stressed with gene transfer protocols via viruses, chemical transfection reagents, or physical methods, gene-of-interest (GOI) expression and viable cell recovery plummets. In the case of genome integrating vectors like lentivirus or retrovirus, it’s noted that the FDA prefers fewer than five copies of the transgene per cell to minimize risk of oncogenic transformation. 3 So, where to find a clinic-friendly reagent that could reduce multiplicity of infection to less than five and/or reduces costly dose-per-cell of the virus or plasmid?
Enter RoosterGEM. RoosterGEM is a complete genetic engineering medium, which means that it can be used in lieu of MEM (or the equivalent) during chemical transfection or transduction. It allows investigators to lower the costs of their vector material and improves transfection/transduction efficiency while maintaining high cell viability and the nominal cell phenotype. The product also is very adaptable to bioprocesses where cells are genetically modified before a large cell expansion (i.e., with integrating lentivirus or transposon)—or after a cell expansion (i.e., with a transiently expressed gene via mRNA, AAV, or adenovirus). There are no licensing strings attached. And finally, RoosterGEM is now available in a clinically translatable cGMP format.
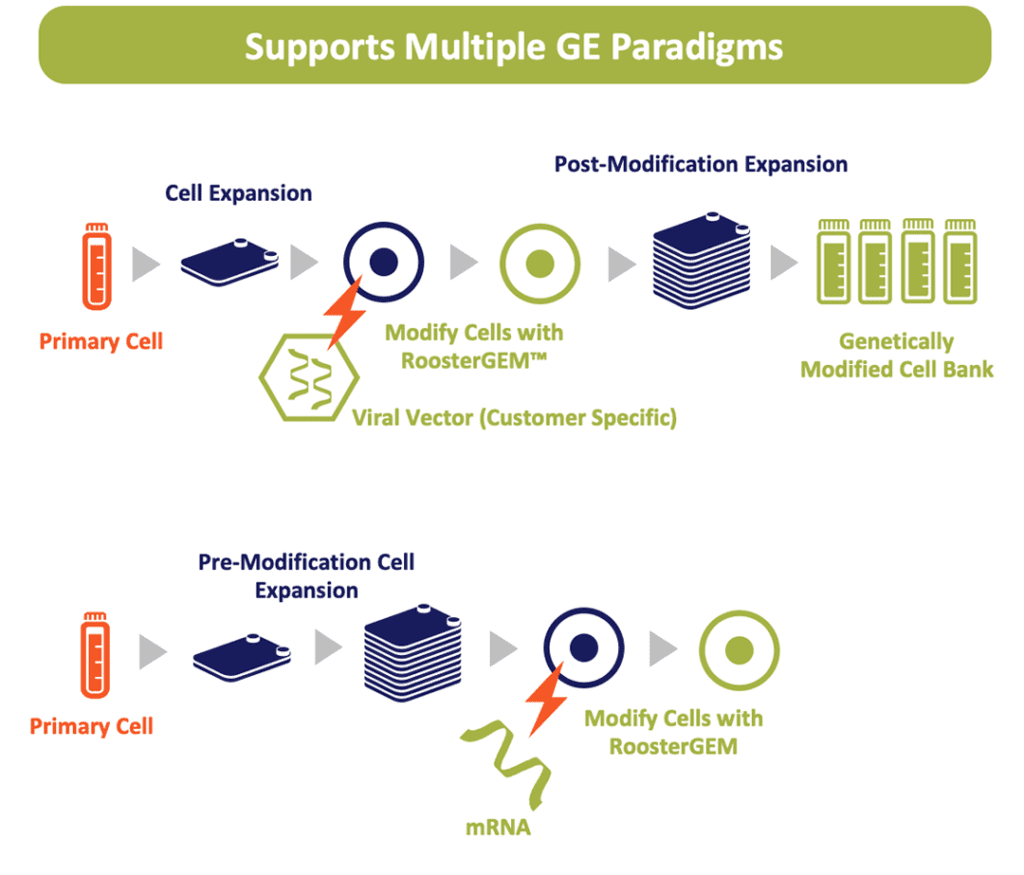
Figure 3. RoosterGEM can be used to simplify and streamline bioprocess to bring gene-modified primary cells into the clinic. It could be used with integrating vectors (above) where gene transfer is performed prior to an expansion of cell doses, or in uses where the vector is transiently expressed (below), such is with mRNA transfection.
Product & Process Optimized for Gene Transfer
Next, Terri Willstaedt took the reins of this webinar to describe recent work on how RoosterGEM facilitates critical process parameters for scale-up with RoosterBio’s premier cell type of interest, mesenchymal stromal/stem cells (hMSCs). Willstaedt showed that despite low MOI (=2), lentiviral transfection efficiency of the zsGreen reporter gene with human umbilical cord and bone marrow donors (3 each) remained quite high (40-80%), depending on tissue origin and donor), when transduction was with RoosterGEM. The use of lower MOI assures lower integrated copy numbers per transduced cell, minimizing the chance of genotoxic hazards. Incidentally, we also tested different promoters (EF1A, CMV, or CAG) to determine which lentivector (LV) performed most efficiently in BM-hMSCs across different MOIs and two LV vendor sources. In a nutshell, if the higher quality LV prep is used, the EF1a promoter yielded high transduction efficiency (~75-80%) at an MOI of two—comparable to promoters constructed with viral sequences (CMV or CAG). This is important because long-term silencing of CMV and CAG can occur, and thus EF1a may be preferable.
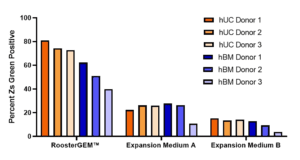
Figure 4. At very low MOI (=2), RoosterGEM boosts lentiviral transduction efficiency (% ZsGreen + Cells) of cells from six tissue donors (hUC 1, hUC2, hUC 3, hBM 1, hBM 2, hBM 3) and two tissue types (umbilical cord or bone marrow) in comparison with two formulations of conventional expansion media.
Willstaedt then demonstrated a process where expression of a RoosterGEM-assisted LV transduction with a zsGreen reporter remained stable (~95% GFP+) across 4 passages (or ~18-22 population doublings, PDs) via master cell banks derived from both hBM-MSCs and hUC-MSCs. Symmetric expansion across 18-22 PDs would amount to a fold increase in total cells of more than 105—or 1011 cells—if begun from a seed population of 1 million transduced MSCs; that translates to possibly hundreds of doses of genetically engineered MSCs for a human trial.
Do cells genetically engineered with a RoosterGEM process still retain their parental phenotype and key MSC features after RoosterGEM transduction? Yes. INFγ-stimulated indoleamine 2, 3- dioxygenase (IDO) activity remained the same, irrespective of LV transduced cell type (hBM- and hUC-MSCs); this is a benchmark of hMSC immunomodulatory activity. The panel of angiogenic cytokines and factors also remained constant whether -/+ RoosterGEM; this suggests maintenance of MSCs’ innate wound healing capacity. Furthermore, regardless of treatment or tissue origin, all cell types could demonstrate trilineage differentiation (into bone, fat, or cartilage) and could maintain the classic surface markers signature of MSCs (-CD34, -CD14, -CD45 and +CD90, +CD166, +CD73, +CD105).
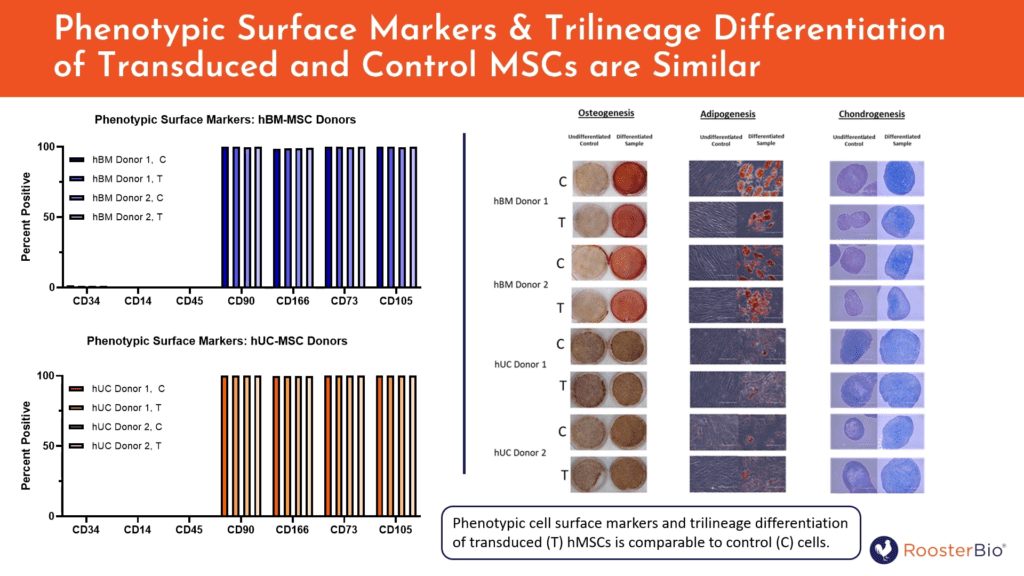
Figure 5. Cells transduced with ZsGreen-expressing lentivirus using RoosterGEM exhibit the same properties of their untransduced parental cells, including a panel of putative MSC surface markers (-/+, left) or trilineage differentiation (right).
After doing much of the initial characterization with hMSCs, Willstaedt explained that it could then be important to see if RoosterGEM’s basic mechanism was generalizable to other useful cell types, i.e., T cells. Under non-optimized culture and cell source conditions at very low MOI (=2), RoosterGEM does in fact increase % transduction of T cells relative to regular T cell media. T cells are, of course, the cell type for CAR-T therapies now marketed today and achieving cures for lymphomas. Similarly, RoosterBio has successfully tried RoosterGEM on NK cells (data not shown), and is expanding the repertoire of use cases and SOPs for other cell types of value to advanced therapies.
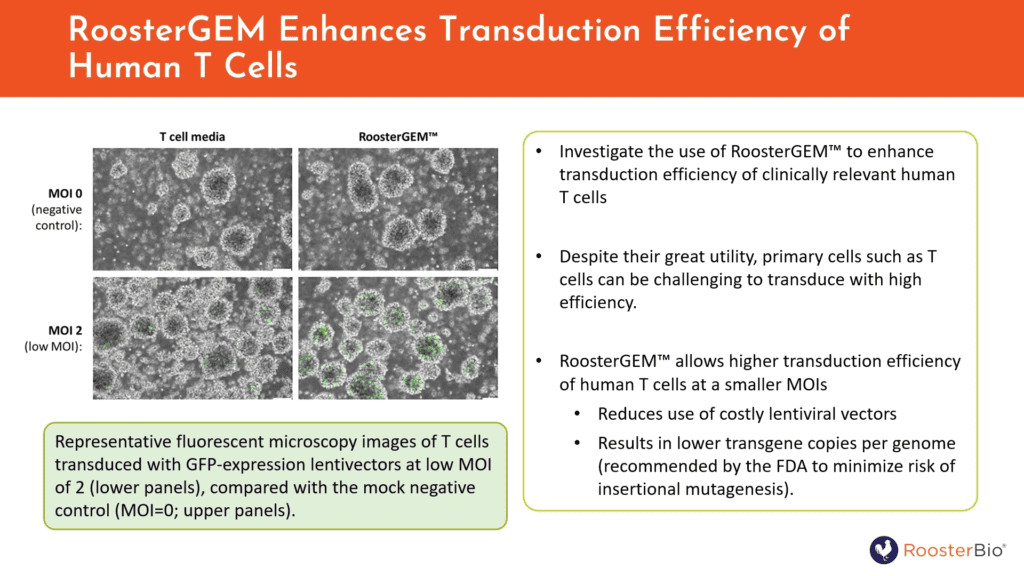
Figure 6. RoosterGEM improves the efficiency of lentivirus transduction of primary T cells vs. cells cultured in regular T cell media.
Beyond Lentivirus: Expanded Uses for RoosterGEM
Because RoosterGEM’s underlying mechanism appears to be broadly applicable across different cell types, we thought that it might also be compelling to observe whether the reagent could be applied with various popular gene transfer techniques outside of lentiviral transduction. Dr. Mary Doolin spoke about this expanded utility following Terri Willstaedt’s report in the webinar.
Adeno-associated virus (AAV) is a common gene platform for several clinical products administered to patients in vivo. However, AAV might also be considered an attractive option for developers of ex vivo gene modified cell therapies who want to express the therapeutic GOI transiently instead of permanently as per LV. In addition, the packaged AAV genome is single-stranded DNA, making it an ideal template of donor DNA for homologous recombination-mediated gene edits or knock-ins in concert with CRISPR-Cas9, zinc finger nucleases, TALENs or meganucleases. We therefore tested a variety of serotypes to determine whether AAV transduction could be enhanced by RoosterGEM. Dr. Doolin observed that AAV serotypes -DJ and -6 will best transduce hMSCs, and that RoosterGEM improves transduction of these and other serotypes at the lower titer (MOI 7.3e3). This result can help investigators save on costs of AAV stocks for prospective ex vivo gene modified cell therapies. Used with RoosterGEM, it would be interesting to see if recombinant AAV could be used to perform targeted gene knock-ins with high efficiency. 4, 5 As this ongoing work continues, it will also be fascinating to report how other viral modalities can be assisted by RoosterGEM transduction, such as adenovirus and baculovirus.
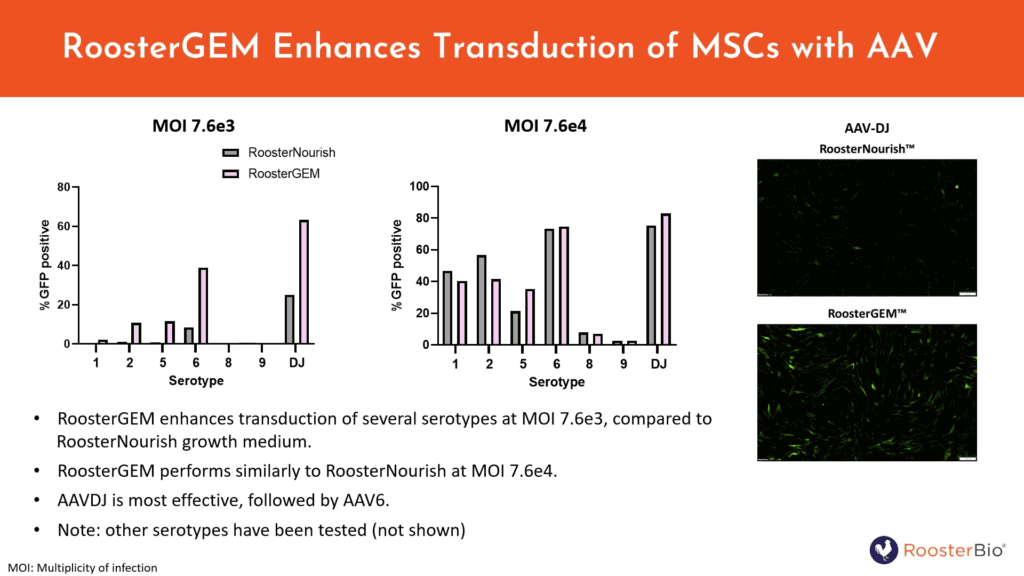
Figure 7. RoosterGEM can increase AAV hMSC transduction efficiency of the GFP reporter at sub-maximal MOIs, particularly with AAV-DJ and AAV-6 serotypes.
The first parts of the webinar focused on viral transduction. Doolin’s next segment described recent efforts to enhance the chemical transfection of hMSCs with mRNA or plasmid DNA (pDNA). pDNA can be expressed transiently in cells, or it can be integrated for permanent expression via transposases like Sleeping Beauty or piggyBAC, or via serine recombinases that use psedudosites or prepositioned attSites. mRNA is expressed transiently but could encode targeted nuclease activities for permanent genome edits. Obviously, the capacity for mass production of mRNA intended for human doses has increased dramatically since 2020—and with these economies of scale—manufacturing costs per dose can be as low as ~$1 per 100 micrograms.
Because of the transient expression afforded by mRNA and pDNA, Doolin recommends a biomanufacture approach to genetically modify hMSCs that’s a distinct alternative to LV’s schema. That is, not only is expression with pDNA and mRNA quite temporary, there’s also dilution in expression output with each cell division across passages. It thus becomes necessary to first expand the seeded cells to a desirable number before cell transfer to the final biomanufacturing step. Doolin showed that this is achievable with the help of RoosterGEM. First, we observed that with RoosterGEM, cells are successfully lipofected after robust expansion out of a smaller seed density plating across three days. Hence, you don’t have to transfect within 12-24h after plating as per conventional protocols. Next, consistent with the transience of pDNA or mRNA, we observed that cells should not be diluted with more passages with pDNA or mRNA.
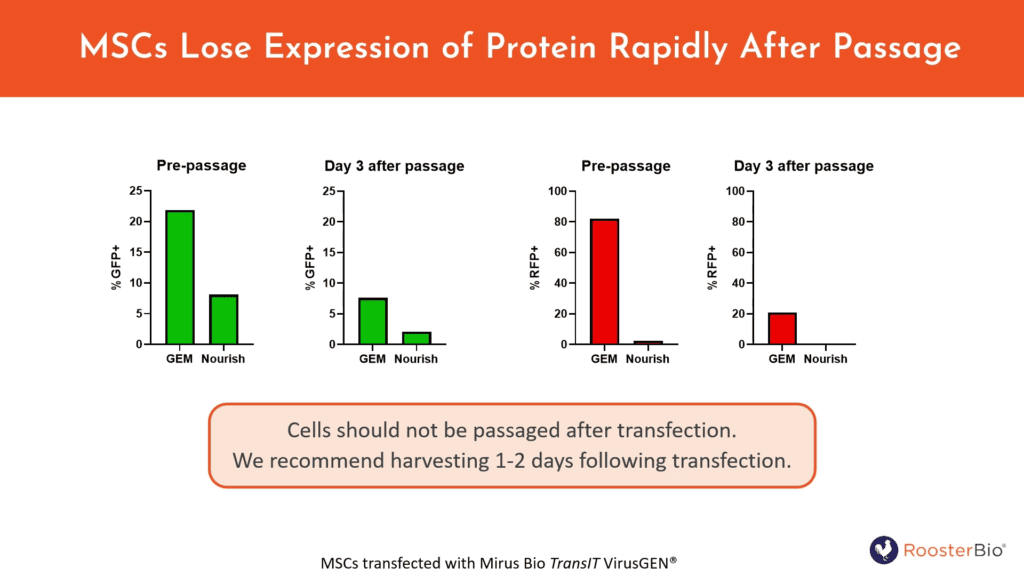
Figure 8. Cells should not be passaged after transfection of transiently expressing GOIs, as shown by diminishing GFP+ cells transfected with pDNA and RFP+ cells transfected with mRNA (-/+ RoosterGEM) 3 days after a passage. Note the boost in transfection efficiency conferred by RoosterGEM to hMSCs. However, even with RoosterGEM, we recommend harvesting 1-2 days following transfection.
A variety of chemical transfection methods and reagents all confirm the usefulness of RoosterGEM to enhance performance on difficult-to-transfect hMSCs. We worked with two companies’ reagent systems that offer a path to GMP-compatible manufacture of their transfection reagents: (a) Polyplus’ cationic polymer reagent that is chemically defined (CD) and animal component-free (XF) and (b) Mirus Bio’s TranIT VirusGEN® that’s also CD and XF. We also tested (c) Vernal Biosciences’ LNP formulation with RoosterGEM; vernal offers cGMP mRNA synthesis for its customers. In all examples, RoosterGEM dramatically increased the fraction of transfected cells, typically above 80%.
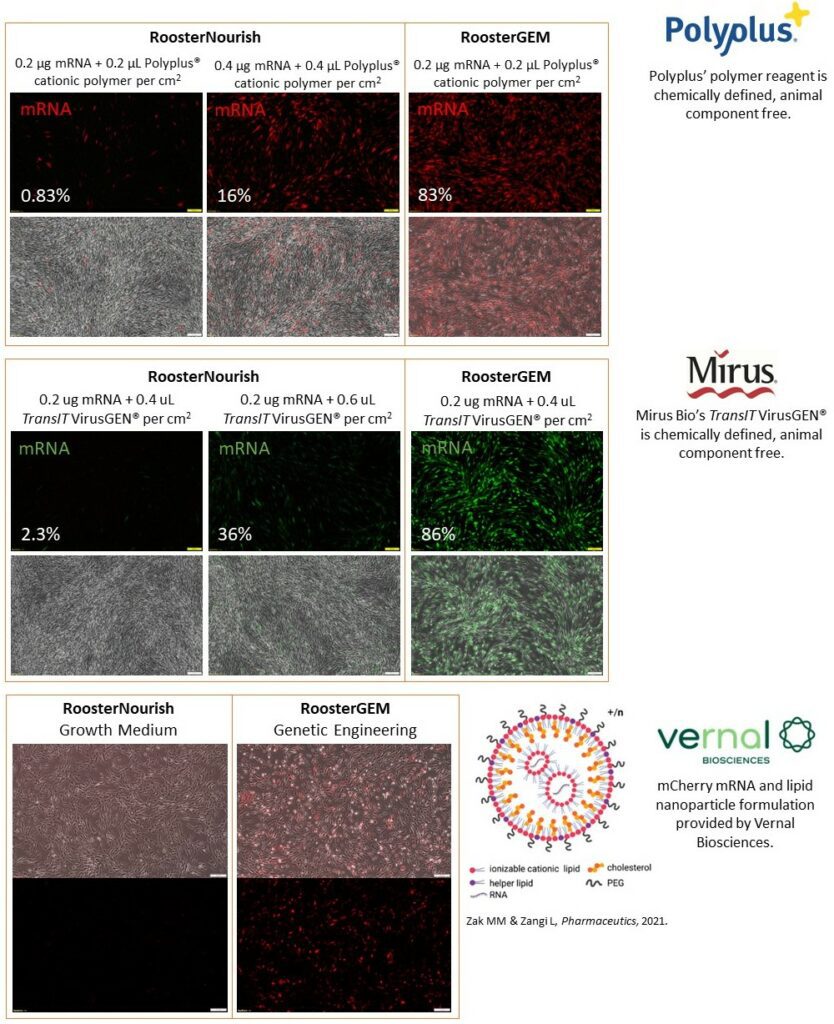
Figure 9. RoosterGEM improves mRNA “transfectability” of hMSCs as quantified by flow cytometry and shown here by fluorescence microscopy images. mRNA encoding RFP with Polyplus cationic polymers (Panel A), mRNA encoding GFP with Mirus Bio’s TransIT VirusGEM® (Panel B), and mRNA encoding RFP with nanoparticles from vernal Biosciences’ specially formulated LNP (Panel C) all exhibited marked increase in transfection efficiency with RoosterGEM vs. normal growth medium (RoosterNourish™).
The Gene Transfer Odyssey Continues…
We greatly look forward to finding where else RoosterGEM can best fit into additional gene transfer applications, with the ultimate goal being to lower the barrier of entry for novel genetically modified cell therapies in the clinic. This blog is merely a summary of our well-viewed webinar, so please be sure to watch it in its entirety to capture the full download of knowledge. While you’re watching, we at RoosterBio are actively seeking new ways to translate this unique reagent into our customers’ success stories. Is there more to show related to different viruses, siRNA/RNAi, RNPs with CRISPR-Cas9, new cell types, and scalable bioprocess? Stay tuned! A new chapter in our gene transfer Odyssey is being written already.
References
- Virgil Aeneid Book II. https://www.poetryintranslation.com/PITBR/Latin/VirgilAeneidII.php#_Toc536009309.
- Grupp, S. A.; Kalos, M.; Barrett, D.; Aplenc, R.; Porter, D. L.; Rheingold, S. R.; Teachey, D. T.; Chew, A.; Hauck, B.; Wright, J. F.; Milone, M. C.; Levine, B. L.; June, C. H., Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 2013, 368 (16), 1509-1518. 10.1056/NEJMoa1215134
- Zhao, Y.; Stepto, H.; Schneider, C. K., Development of the First World Health Organization Lentiviral Vector Standard: Toward the Production Control and Standardization of Lentivirus-Based Gene Therapy Products. Hum Gene Ther Methods 2017, 28 (4), 205-214. 10.1089/hgtb.2017.078
- Meshitsuka, S.; Ninomiya, R.; Nagamura-Inoue, T.; Okada, T.; Futami, M.; Tojo, A., CRISPR/Cas9 and AAV mediated insertion of beta2 microglobulin-HLA-G fusion gene protects mesenchymal stromal cells from allogeneic rejection and potentiates the use for off-the-shelf cell therapy. Regen Ther 2022, 21, 442-452. 10.1016/j.reth.2022.09.009
- Srifa, W.; Kosaric, N.; Amorin, A.; Jadi, O.; Park, Y.; Mantri, S.; Camarena, J.; Gurtner, G. C.; Porteus, M., Cas9-AAV6-engineered human mesenchymal stromal cells improved cutaneous wound healing in diabetic mice. Nat Commun 2020, 11 (1), 2470. 10.1038/s41467-020-16065-3
