Listen to this Blog:
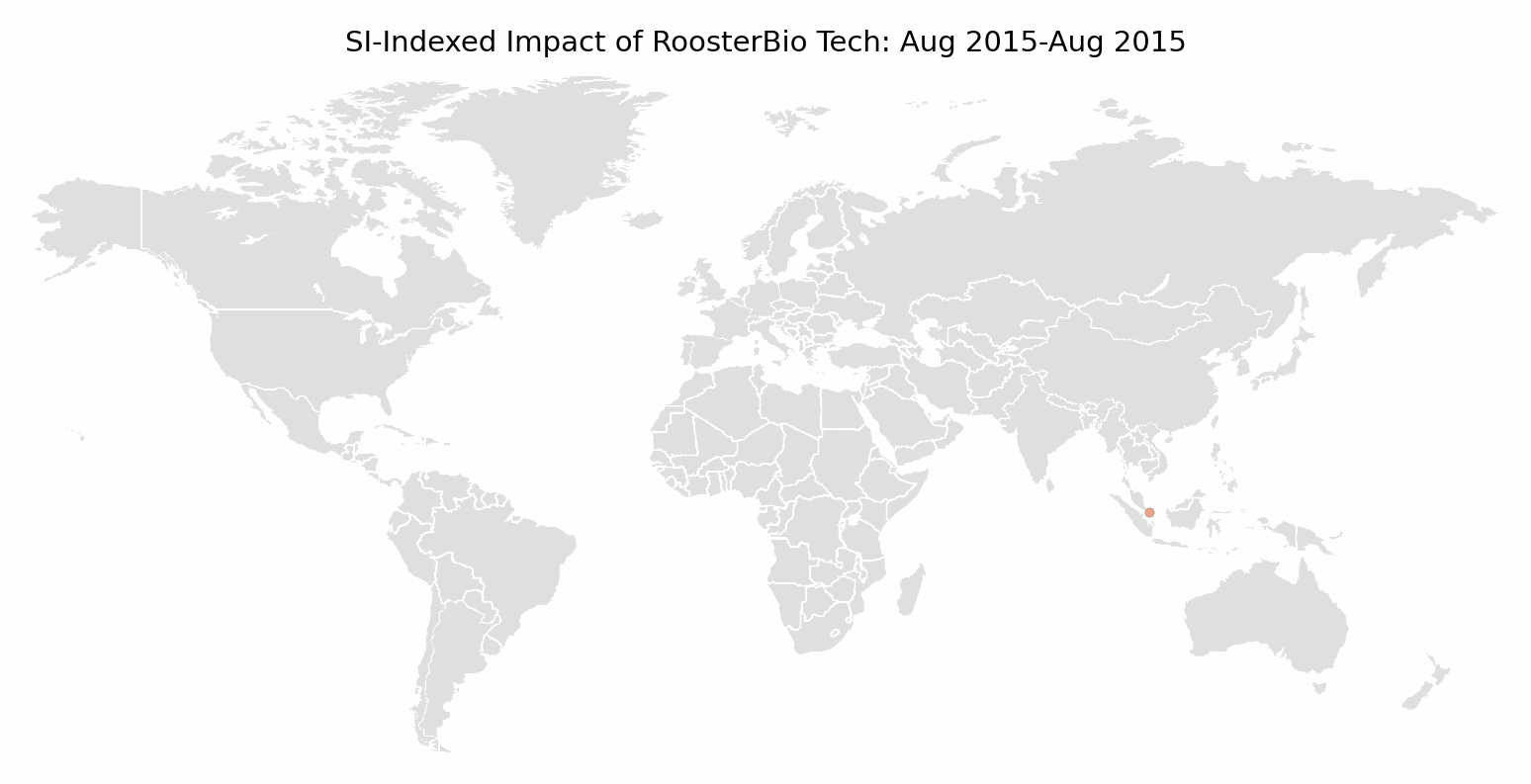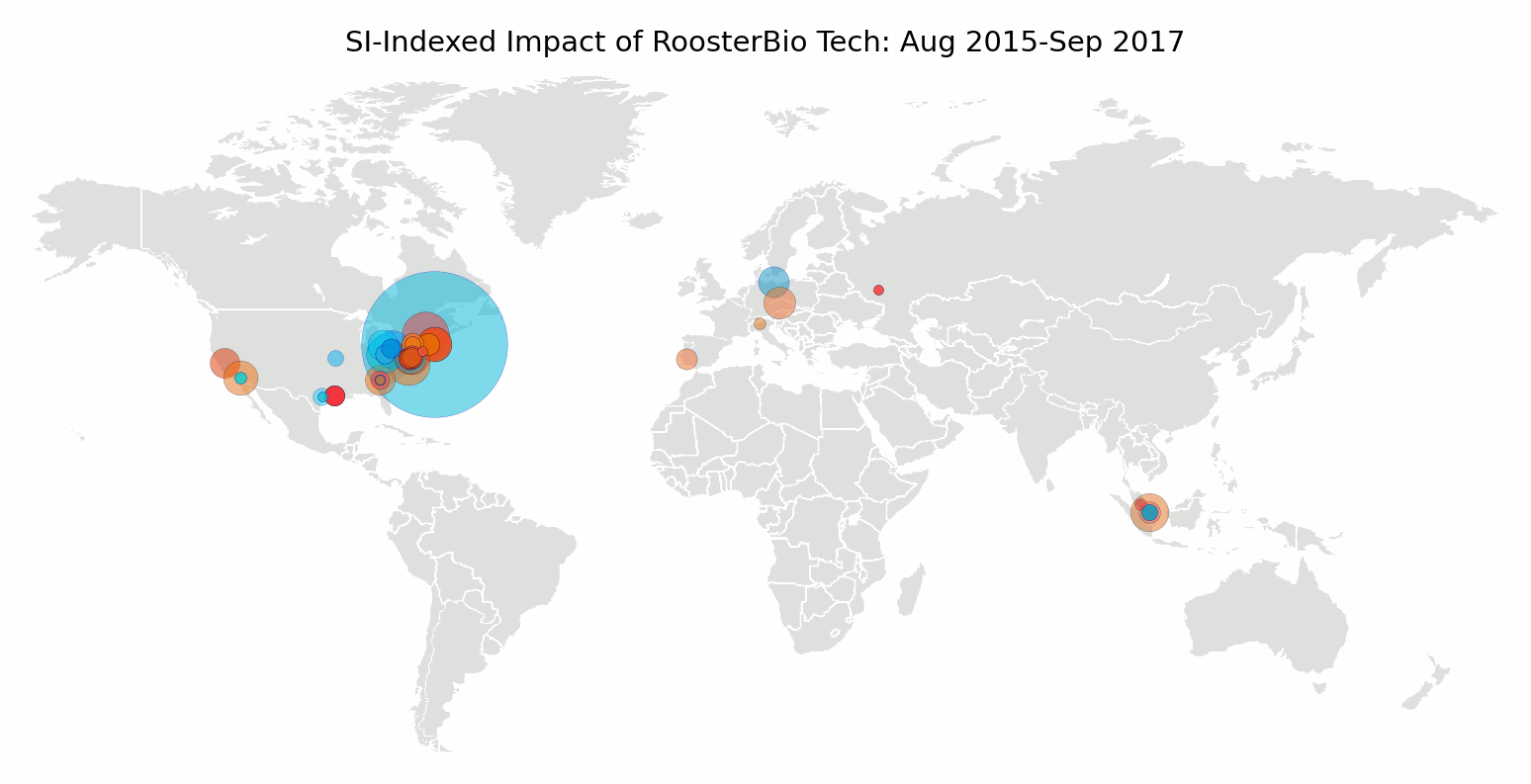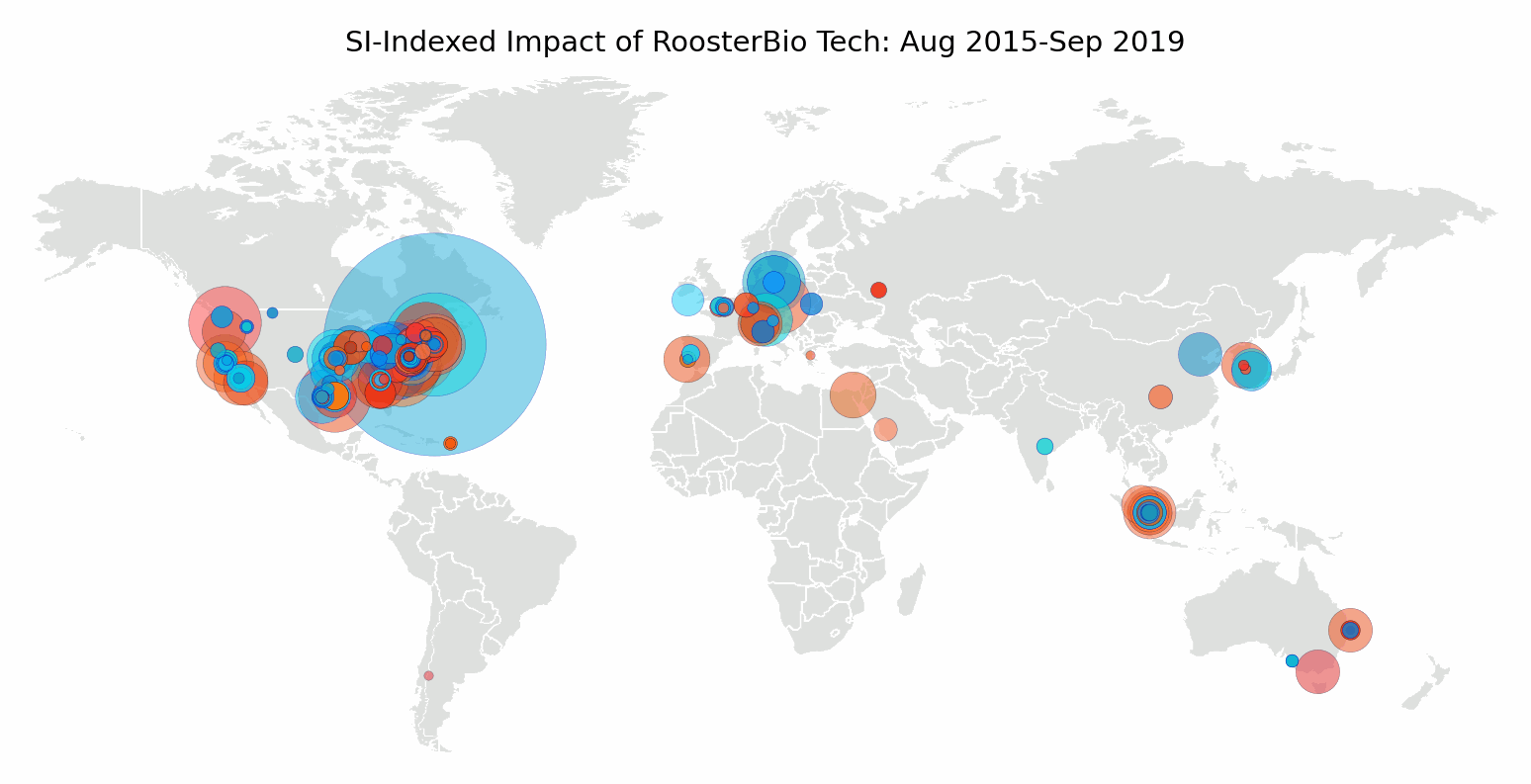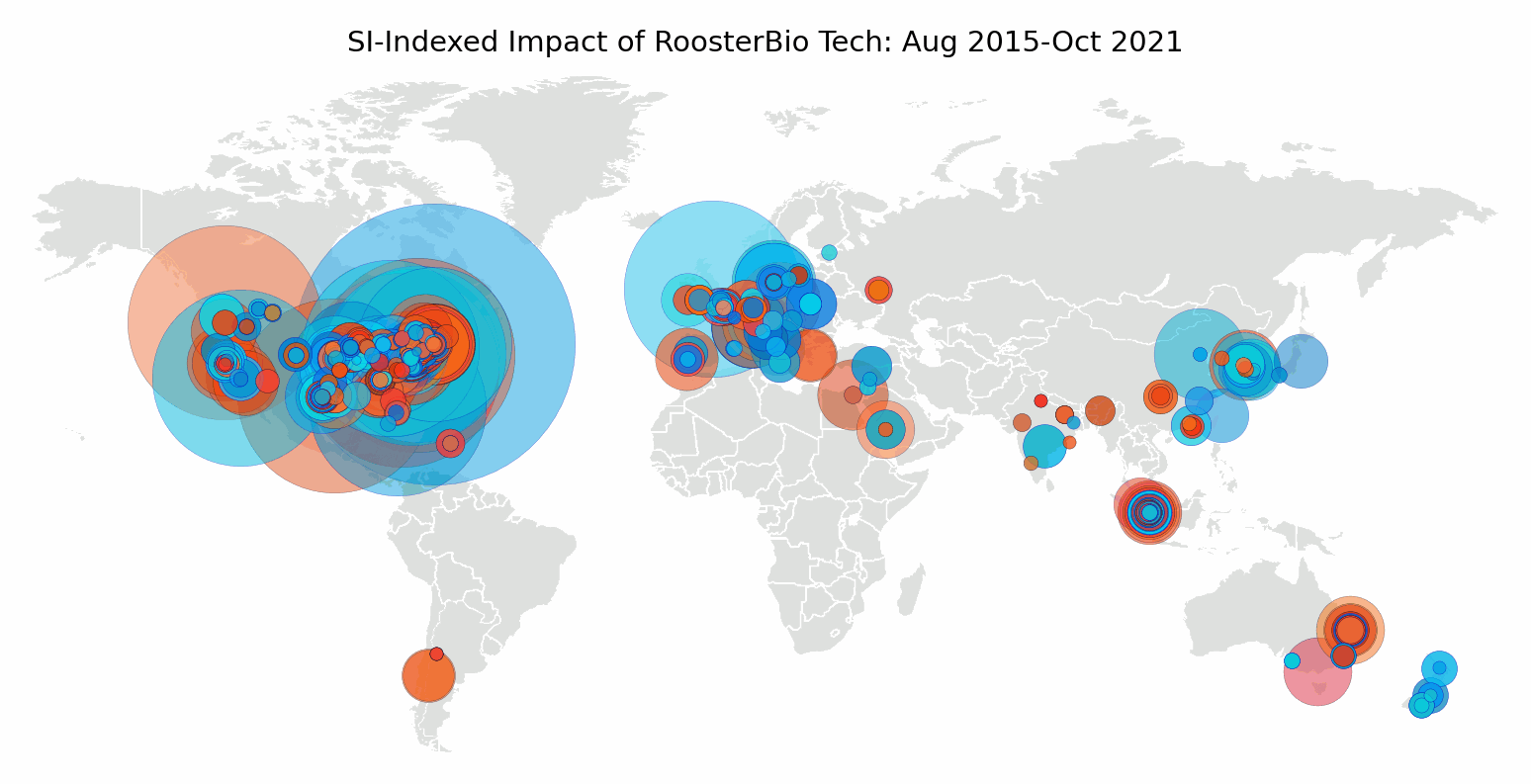
Let’s agree — Healthcare innovations require infrastructure, but the infrastructure to support the innovations of cell therapy needs shoring up. That is, living cells must be commercially available as a predictable raw material for future breakthroughs in the cellular therapy community and the rapidly maturing field of regenerative medicine. Industrialized quality control establishes a reliable process between researcher, regulator, and clinician. In addition to safety and efficacy in experimental trials, cellular manufacturing must be cleared of bottlenecks to meet demand on a commercial scale. Patient populations could be millions of people (cancer[1]) to tens of millions (diabetes[2] or arthritis[3]) in the United States alone.
RoosterBio accepted the challenge and built a business to enable a system of high quality and scalable volumes (billions) of human mesenchymal stromal cells (hMSC), paired media systems, and Good Manufacturing Practice (GMP) bioprocess expertise. [4] Cell suppliers — like RoosterBio — absorb the ambiguity of hMSC sourcing and enable researchers to focus on excavating their own domain expertise, which further adds to the predictability of the final therapy. Together, the elements of this system aim to simplify and streamline the workflow for basic research and clinical translation, alike.
But, does it work? Are tractable cellular therapies being built on the foundation of an hMSC standard? We took a peek into the ivory tower through the corpus of peer-reviewed publications to understand who used RoosterBio hMSCs and to what pre-clinical end. Affirming our casual observations, our deeper look identified support for clinical-grade hMSC standards in reviews, with hundreds of papers across subject domains citing the technology, and a geographically extended community brought together by a commercially available cellular standard.
Our Trip Down Memory Lane
RoosterBio opened its lab doors in the fall of 2013. In August 2015, the Agency for Science, Technology, and Research (A*STAR) described RoosterBio bone marrow hMSC as fast-growing in serum-free media, as published by Cytotherapy. [5] A few months later, a team from the University of Vermont introduced a self-healing hydrogel formulated for cell transplantation using RoosterBio hMSC in the journal Biomacromolecules. [6] That year, RoosterBio hMSCs were earning their place among academic investigators as “go-to” hMSCs for experiments that required standard-setting, high-performance cells. Since then several hundred papers have cited RoosterBio technology, accumulating more than 5000 citations from institutions around the world.
We mapped author affiliations of publications citing RoosterBio cells or media. We found global adoption, which is an encouraging outcome for an hMSC standard. A consistent, well-defined hMSC and media system serves as a benchmark to compare experiments between researchers, research groups, and in a similar testbed, over time. Even teams collaborating on a single paper span continents. Coauthors came together from the University of Pittsburgh, Royal College of Surgeons in Ireland, and the Indian Institute of Technology Guwahati to engineer a scaffold for vascular tissue engineering using particles released by primary adipose-derived hMSC. [7]
RoosterBio cannot and would not claim responsibility for our customer’s breakthroughs, but we can celebrate them. We can also facilitate data reproducibility by democratizing the same raw materials as off-the-shelf products for use by many. The two highest cited papers (to date) using RoosterBio technology helped make the leap from millimeter to macroscale in the field of tissue engineering. One comes from Wyss Institute and the other from Rice University.
A Success Story in 3D
3D printed tissues were hitherto limited to mouse-model size because cells inside a solid block did not receive a consistent supply of nutrients. Dr. Jennifer Lewis and her team at the Wyss Institute figured out how to embed an open network of channels in a >1cm wide construct embedded with living cells. [8] Their construction and environmental control successfully guided hMSC towards osteogenic lineage for more than 6 weeks. This removes a major stumbling block to build true-to-size organs for the tissue engineering community. Her paper has earned nearly 1000 citations, which emphasizes the traction and importance across the academic community of this contribution.
One of those citations came from Rice University Professor, Jordan Miller. He assembled a coalition of experts across the United States and built multiple intertwined (not intersecting) biomaterial vessel-like passageways. [9] They reverse-engineered the topology of the discrete, nested systems that carries your blood, air, and lymph. The complexity of the problem is reflected in the diversity of the team: engineers, cell biologists, pathologists, computational scientists, and artists. Their achievement became the foundation for a patent and the Houston-based company, Volumetric Biotechnologies. As full-scale 3D printed organs come online, experts estimate more than 6 trillion cells will be needed to lab-make a replacement liver for those on the donor waiting list, [10] and hMSCs could be important component raw materials. [11] An industrialized supply chain must be in place to realize the potential of these breakthroughs. Experts hypothesize demand will peak at 64 trillion MSC annually in 2040 for organ manufacturing alone. Read more about the vast potential scale of the MSC opportunity space. [10]
Predicting the In Vivo Future
Beyond tissue engineering and acquisition of industrial know-how, highly cited papers from RoosterBio customers illuminate a responsible path to clinical trials with analytical tools and mechanistic understanding. For example, a growing body of evidence points to the repertoire of inter-cellular signaling factors produced by the cell — not necessarily the cell itself — as the source for many hMSC therapeutic effects. Dr. Stella Kourembanas took a step back from the clinic to the lab and chronicled the cause-and-effect relationships between macrophage, inflammation, and restricted lung growth in preterm infants. [12] With her Boston Children’s Hospital and Harvard Medical School team, Kourembanas defined the therapeutic capacity of hMSC exosomes to guide their appropriate use. Learn more about exosomes and their clinical relevance.
Similarly, the US Food and Drug Administration built an analytical tool to correlate cytokine stimulation with immune suppression using bone marrow hMSC, because clinical results were inconsistent. [13] The international community agrees. A review authored by experts from Beijing Cancer Hospital and The University of Iowa calls for a standard clinical grade hMSC to reduce batch-to-batch variation and phenotypic diversity. [14] These three papers on mechanism and standardization reflect the consensus of the community, as each has more than 100 citations to date.
The Far-From-Standard Path Ahead
Noticeably absent is a preponderance of highly cited studies injecting a loose suspension of hMSCs into an injury site. If autologous donation and injection worked reliably, then it would be baked into the suite of treatments used clinically. The way forward just isn’t that simple, which is why scaffold guided tissue engineering, embedded cell encapsulations, exosome research, and advances in material science are humbling, thrilling, and worthy of great effort. It seems likely that better questions informed by fundamental science will gravitate clinicians to the right medicine for the right patients, as presented in this blog, MSCs & Precision Medicine. There is no silver bullet solution, but experience shows us that the effervescent combination of biology, chemistry, and physics expertise can spin off breakthroughs rapidly — and in surprising new directions — during a technology’s “log phase.”
Academia’s “Ivory Tower” (often unintentionally) keeps many secrets buried under deep domain expertise, above steep learning curves, and sometimes behind paywalls. Yet, when developed in an interdisciplinary manner, their knowledge and creativity become transformed into novel and useful technology, which becomes foundational as a breakthrough product to enrich our lives via welcome and bold ways — including towards our own healthspans.
In the last few years, clinical-grade hMSCs broke free of the lab and are now characterized to the point that they can be integrated into a supply chain — a broader community involving not just white coats, but hard hats too. The next generation of cell therapy can thus build on a reliable foundation of hMSC standards. Our question becomes: how to turn knowledge into a process and into a treatment. It could be as easy as A – B – standardize the hMSC.
References
1. Cancer Stat Facts: Common Cancer Sites Available from: https://seer.cancer.gov/statfacts/html/common.html#:~:text=In%202021%2C%20roughly%201.9%20million,the%20most%20common%20cancer%20diagnosis.
2. CDC.gov. National Diabetes Statistics Report 2020 2020; Available from: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf.
3. CDC.gov. National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP) Available from: https://www.cdc.gov/chronicdisease/resources/publications/factsheets/arthritis.html.
4. Olsen, T. R. and J. A. Rowley, Corporate profile: RoosterBio, Inc. Regen Med, 2018. 13(7): p. 753-757. 10.2217/rme-2018-0092 https://doi.org/10.2217/rme-2018-0092
5. Tan, K. Y., et al., Serum-free media formulations are cell line-specific and require optimization for microcarrier culture. Cytotherapy, 2015. 17(8): p. 1152-65. 10.1016/j.jcyt.2015.05.001 https://doi.org/10.1016/j.jcyt.2015.05.001
6. Miao, T., et al., Self-Healing and Thermoresponsive Dual-Cross-Linked Alginate Hydrogels Based on Supramolecular Inclusion Complexes. Biomacromolecules, 2015. 16(12): p. 3740-50. 10.1021/acs.biomac.5b00940 https://doi.org/10.1021/acs.biomac.5b00940
7. Cunnane, E. M., et al., Extracellular Vesicles Enhance the Remodeling of Cell-Free Silk Vascular Scaffolds in Rat Aortae. ACS Appl Mater Interfaces, 2020. 12(24): p. 26955-26965. 10.1021/acsami.0c06609 https://doi.org/10.1021/acsami.0c06609
8. Kolesky, D. B., et al., Three-dimensional bioprinting of thick vascularized tissues. Proc Natl Acad Sci U S A, 2016. 113(12): p. 3179-84. 10.1073/pnas.1521342113 https://doi.org/10.1073/pnas.1521342113
9. Grigoryan, B., et al., Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science, 2019. 364(6439): p. 458-464. 10.1126/science.aav9750 https://doi.org/10.1126/science.aav9750
10. Olsen, T. R., et al., Peak MSC-Are We There Yet? Front Med (Lausanne), 2018. 5: p. 178. 10.3389/fmed.2018.00178 https://doi.org/10.3389/fmed.2018.00178
11. Ramachandran, S. D., et al., In Vitro Generation of Functional Liver Organoid-Like Structures Using Adult Human Cells. PLoS One, 2015. 10(10): p. e0139345. 10.1371/journal.pone.0139345 https://doi.org/10.1371/journal.pone.0139345
12. Willis, G. R., et al., Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am J Respir Crit Care Med, 2018. 197(1): p. 104-116. 10.1164/rccm.201705-0925OC https://doi.org/10.1164/rccm.201705-0925oc
13. Klinker, M. W., et al., Morphological features of IFN-gamma-stimulated mesenchymal stromal cells predict overall immunosuppressive capacity. Proc Natl Acad Sci U S A, 2017. 114(13): p. E2598-E2607. 10.1073/pnas.1617933114 https://doi.org/10.1073/pnas.1617933114
14. Yin, J. Q., J. Zhu, and J. A. Ankrum, Manufacturing of primed mesenchymal stromal cells for therapy. Nat Biomed Eng, 2019. 3(2): p. 90-104. 10.1038/s41551-018-0325-8 https://doi.org/10.1038/s41551-018-0325-8
