In 2022, it’s still early morning in Gene and Cell Therapy Land. Behold! There’s “crowing” interest in the deployment of human mesenchymal stromal/stem cells (hMSCS) as cellular delivery systems for gene therapies. [1] Like the CAR-T technologies that drove ex vivo T cell therapies to an inflection point in 2013, [2, 3] it’s plainly obvious that genetic engineering could likewise leverage a huge force multiplier for greater clinical efficacy with hMSCs.
Why? The mammalian cell can be imagined as a computational network [4, 5] that can be programmed with artificial gene sequences to sense its environment, provide stability for controlled release of therapeutic cargoes, and ameliorate disease locally or systemically for a determined amount of time. hMSCs have demonstrated a consistent safety record across hundreds of clinical trials and thousands of patients worldwide; the very first marketed hMSC therapies are now available outside the USA. Before gene-edited and/or modified hMSC therapies are widespread, however, there’s a nagging old problem that we need to solve: how to efficiently deliver the gene cargoes into cells. [6] Being primary cells, hMSCs are not as easy to transfect or transduce as less-clinically translatable cell lines—like HeLas or HEKs!
Luckily, RoosterBio has been hard at work to provide one solution. Our complete genetic engineering medium, RoosterGEM™, demonstrates greatly enhanced efficiency for lentiviral [7] transduction and mRNA transfection [8] into adherent cells like hMSCs—and other cell types too.
This RoosterGEM reagent can assist in at least two ways. First, the lab or manufacturing center can conserve its resources, burning through a pricey stock of GMP virus titer or nucleic acid much more slowly. Second—with fewer MOIs, that means a potentially lower load of excipient viral material that hitches a ride into the final, vialed cell product—either “on” the cells or “in” them. A gentler sprinkling of capsids might thus plausibly translate to lower immunoreactivity for a less “spicy” (i.e., inflammatory) therapy, and possibly, longer duration benefit or safety. [9, 10, 11] If you wish to use RoosterGEM as an ancillary material for clinical trials, no problem; its release in a GMP product format is imminent for early 2023—and there are no licensing “strings” attached.
Does it make sense to try RoosterGEM on additional gene transfer modalities beyond lentivirus or chemical transfection? We certainly think so. One such notable system is adeno-associated virus (AAV), the basis for FDA-approved gene therapies Luxturna (Spark Therapeutics) and Zolgensma (Novartis Gene Therapies). AAV can be already administered safely and efficaciously directly to humans in vivo, without an ex vivo prepared cell “carrier.” However, plausible benefits of AAV’s use in concert with hMSCs may include some of these key points:
- Situations where permanent genetic modification via chromosomal integration is undesirable, where AAV can deliver longer-lasting yet still transient and doseable therapeutic effect (e.g., days of expression translating to months of benefit) via transduced hMSCs [12, 13, 14]
- A growing, reliable network of CRO/CDMO expertise and capacity is facilitating scalable, GMP-grade manufacture of AAV and other secreted biologics for many cell & gene therapy developers [15, 16]
- AAV’s compact (~4.5kb) payload size enforces a simplicity into the design of its artificial therapeutic cargoes—smaller size ~ fewer variables to contend with [17]
- AAV’s single-stranded genome is ideal for supplying a long second strand for homologous recombination-mediated genome editing applications (-/+ CRISPRs, zinc finger nucleases, TALENs, or meganucleases) [18, 19, 20, 21]
- When AAV is deployed via ex vivo within cells instead of by direct in vivo means, concerns of capsid immunogenicity and neutralizing antibodies (NAbs) can be mitigated—particularly via hMSCs’ well-known tolerogenic activity and membrane encapsulation of the genetic dose [22]
- hMSCs deployed alongside in vivo administered AAV could also help tolerize tissues to AAV capsids for indications that would otherwise consume massive doses (e.g., Duchenne muscular dystrophy) [23]
- AAV tissue tropism can be readily engineered, whereby AAV capsids could possibly be internalized into exosomes or microvesicles for re-targeting, de-targeting, or immune evasion from NAbs [24]
Thus, hMSCs can get along swimmingly with AAVs because of unique technical opportunities to enhance these cells in gene therapies, to genome edit them as targets for modified MSC biofunction (e.g., deletion of putative coagulation effectors), or to employ them as “CHO-like” bioproducers of custom AAVs—encapsulated within extracellular vesicles (EVs) and/or exosomes churned out by hMSCs.
We’re happy to report our recent RoosterGEM results can help investigators cross what’s been coined a “translational speed bump” for AAV gene therapy on hMSCs [25]. Our initial data not only validate others’ findings of high innate transducibility of hMSCs with the specific AAV-DJ [26, 27] and AAV6 serotypes, [20] but also show substantial and highly promising improvement of AAV transduction overall via RoosterGEM use (Table, below).
| Name | MOI = 10e3 | MOI = 10e4 | ||
| RoosterGEM™ | RoosterNourish™ | RoosterGEM | RoosterNourish | |
| AAV1 | + | + | ||
| AAV2 | + | ++ | ||
| AAV3 | + | ++ | ||
| AAV4 | ||||
| AAV5 | + | + | ||
| AAV6 | ++ | + | +++ | +++ |
| AAV6.2 | ++ | + | +++ | +++ |
| AAV7 | + | + | ||
| AAV8 | ||||
| AAV9 | ||||
| AAVrh10 | ||||
| AAVDJ | +++ | + | +++ | +++ |
| AAVDJ/8 | ||||
| AAVPHP.eB | ||||
| AAVPHP.S | + | + | + | |
| AAV2-retro | ||||
| AAVQuadYF | + | +++ | + | |
| AAV2.7m8 | +++ | +++ | + | |
Table, above, illustrating qualitative effects of RoosterGEM complete gene transfer media (-/+, vs. standard RoosterNourish culture media) on RoosterBio bone marrow hMSCs, screened with a diverse collection of AAV serotypes. Two multiplicities of infection (MOI 10,000 or 100,000) are shown, where high % transfection is depicted by dark green shading and lower % transfection is shaded in light green.
Focusing on a smaller set of serotypes, we then collected fluorescent microscopy images of bone marrow hMSCs transduced with smaller or larger titers of AAV-GFP (MOI ~10e3 and MOI ~10e4), viewing time points on Experiment Days 3 and 4. Cells were either transduced with the AAV in standard culture medium (RoosterNourish) or RoosterGEM medium overnight, then replenished with fresh RoosterNourish. Figure 1 (below) shows representative images collected from cells on Day 2 with the lower MOI 10e3 titer. Clearly, we note serotype-selective expression of the GFP transgene (i.e., AAV6 and AAV-DJ) as well as enhanced transduction efficiency that is attributable to RoosterGEM (bottom panel of Figure 1 compared with top panel).
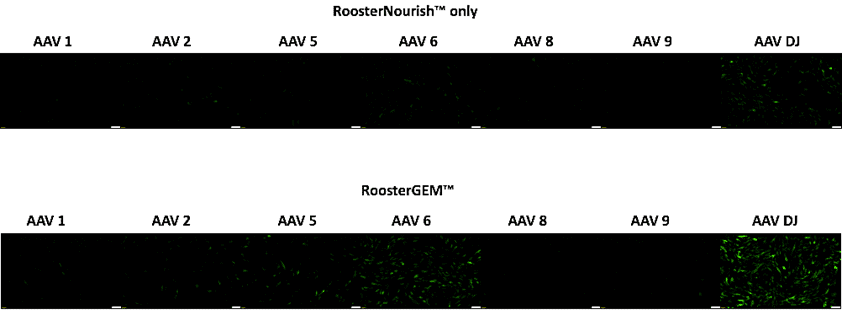
Figure 1, above, representative green fluorescence microscopy images showing GFP transgene expression, comparing transduction via standard RoosterNourish medium (top panel) vs. transduction with RoosterGEM complete gene transfer media (bottom panel) with different AAV serotypes (-1, -2, -5, -6, -8, -9, and -DJ). Note serotype selectivity of -DJ and -6 serotypes for hMSCs and pronounced effect of RoosterGEM on transduction efficiency and level.
How do these qualitative images translate into quantitative Flow data? We then collected Day 3 time point samples transduced with MOI-10e3, and then viewed their % GFP+ expression (Figure 2A, below) and Mean Fluorescence Intensities (Figure 2B, below). What your eye sees in Figure 1 closely resembles population data collected by the flow cytometer instrument; RoosterGEM enhances % transfection to over 60% with the AAV-DJ serotype at this titer. Moreover—consistent with observations shown in the Table (above) taken from our preliminary serotype screen—increasing MOI tenfold to 10e4 will increase the scope of serotypes that can transduce hMSCs (data not shown) and additionally boost the fraction of GFP+ cells in the RoosterGEM + AAV-DJ group to over 80% (data not shown).
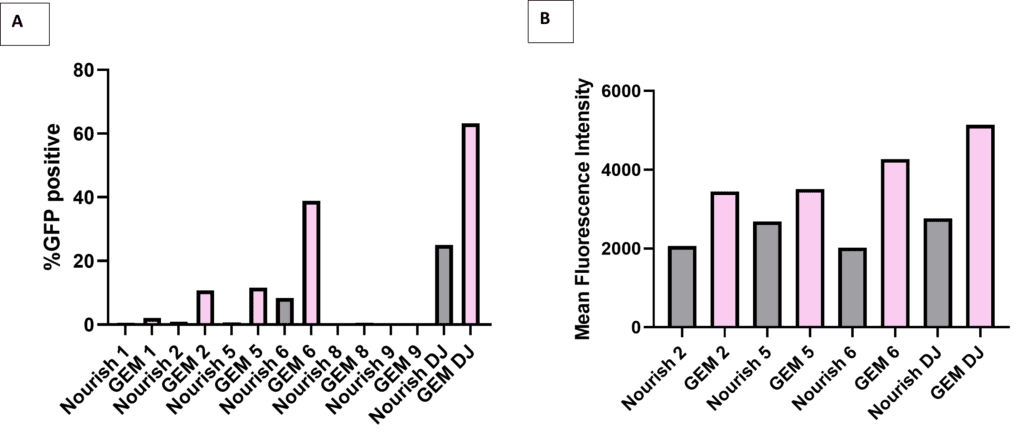
Figure 2, above, flow cytometry data showing % GFP transgene expression (panel A), comparing transduction via standard RoosterNourish medium (grey bars) vs. transduction with RoosterGEM complete gene transfer media (pink bars) with different AAV serotypes (-1, -2, -5, -6, -8, -9, and -DJ) transduced at MOI of 10e3. In Panel B, note parallel increase in RoosterGEM-related mean fluorescence intensity collected for GFP+ cells via serotypes -2, -5, -6, and -DJ.
As this brief blog illustrates, RoosterBio has quickly expanded the range of its RoosterGEM reagent beyond merely lentivirus (the dominant gene delivery system to generate CAR-T therapies). [1] We’re now in the process of demonstrating its value for other transfection/transduction modalities and new adherent and non-adherent cell types of clinical relevance. Importantly, we’re eager to see if future testing could broaden RoosterGEM’s utility into genome editing or “knock-in” applications, [21] techniques that could be greatly enhanced by a recombinant AAV’s homology arms of single-stranded DNA. [20] If a new clinical product is on your mind, remember—a GMP product format for this reagent is just around the corner.
What do you think might be a new use for RoosterGEM? We’re happy to entertain your ideas and expertise, and ever eager to share our protocols and learnings on how we could get it working in your cell and gene therapy lab. Why not contact us to learn more?
References
- Willstaedt, Terri. Genetically Engineered Mesenchymal Stromal Cells (MSCs): A Promising Tool for the Successful Delivery of Therapeutic Genes. 2021; Available from: https://www.roosterbio.com/blog/genetically-engineered-mesenchymal-stromal-cells-a-promising-tool-for-the-successful-delivery-of-therapeutic-genes/.
- Gross, G., et al., Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. Transplant Proc, 1989. 21(1 Pt 1): p. 127-30.
- Grupp, S. A., et al., Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med, 2013. 368(16): p. 1509-1518. 10.1056/NEJMoa1215134
- Mansouri, M., et al., Smart-watch-programmed green-light-operated percutaneous control of therapeutic transgenes. Nat Commun, 2021. 12(1): p. 3388. 10.1038/s41467-021-23572-4
- Zhao, E. M., et al., RNA-responsive elements for eukaryotic translational control. Nat Biotechnol, 2022. 40(4): p. 539-545. 10.1038/s41587-021-01068-2
- Wolff, J. A., The “grand” problem of synthetic delivery. Nat Biotechnol, 2002. 20(8): p. 768-9. 10.1038/nbt0802-768
- Willstaedt, T. M., et al. DEVELOPMENT OF AN OPTIMIZED LENTIVIRAL TRANSDUCTION MEDIUM AND PROCESS TO MANUFACTURE GENETICALLY MODIFIED MSC WORKING CELL BANKS (poster presented at ISCT-2021). Cytotherapy 2022 2022/05/01/ [cited 24 5, Supplement]; S70]. Available from: https://www.sciencedirect.com/science/article/pii/S1465324922002171.
- RoosterBio. RoosterGEM™ Expands into New Fields of “Cell-Grow-Culture” for mRNA Transfection. 2022; Available from: https://www.roosterbio.com/blog/roostergem-expands-into-new-fields-of-cell-grow-culture-for-mrna-transfection/.
- Shao, W., et al., Double-stranded RNA innate immune response activation from long-term adeno-associated virus vector transduction. JCI Insight, 2018. 3(12). 10.1172/jci.insight.120474
- Verdera, H. C., K. Kuranda, and F. Mingozzi, AAV Vector Immunogenicity in Humans: A Long Journey to Successful Gene Transfer. Mol Ther, 2020. 28(3): p. 723-746. 10.1016/j.ymthe.2019.12.010
- Chan, Y. K., et al., Engineering adeno-associated viral vectors to evade innate immune and inflammatory responses. Sci Transl Med, 2021. 13(580). 10.1126/scitranslmed.abd3438
- Stender, S., et al., Adeno-associated viral vector transduction of human mesenchymal stem cells. Eur Cell Mater, 2007. 13: p. 93-9; discussion 99. 10.22203/ecm.v013a10
- Sun, T., et al., Ultrasound-targeted microbubble destruction optimized HGF-overexpressing bone marrow stem cells to repair fibrotic liver in rats. Stem Cell Res Ther, 2020. 11(1): p. 145. 10.1186/s13287-020-01655-1
- Nakajima, M., et al., Mesenchymal Stem Cells Overexpressing Interleukin-10 Promote Neuroprotection in Experimental Acute Ischemic Stroke. Mol Ther Methods Clin Dev, 2017. 6: p. 102-111. 10.1016/j.omtm.2017.06.005
- Srivastava, A., et al., Manufacturing Challenges and Rational Formulation Development for AAV Viral Vectors. J Pharm Sci, 2021. 110(7): p. 2609-2624. 10.1016/j.xphs.2021.03.024
- Alvaro, David and Kelly, Tim. Moving Beyond the Industrialization of MSCs. 2022; Available from: https://www.pharmasalmanac.com/articles/moving-beyond-the-industrialization-of-mscs.
- Elangkovan, N. and G. Dickson, Gene Therapy for Duchenne Muscular Dystrophy. J Neuromuscul Dis, 2021. 8(s2): p. S303-S316. 10.3233/JND-210678
- Russell, D. W. and R. K. Hirata, Human gene targeting by viral vectors. Nat Genet, 1998. 18(4): p. 325-30. 10.1038/ng0498-325
- Bonafont, J., et al., Correction of recessive dystrophic epidermolysis bullosa by homology-directed repair-mediated genome editing. Mol Ther, 2021. 29(6): p. 2008-2018. 10.1016/j.ymthe.2021.02.019
- Srifa, W., et al., Cas9-AAV6-engineered human mesenchymal stromal cells improved cutaneous wound healing in diabetic mice. Nat Commun, 2020. 11(1): p. 2470. 10.1038/s41467-020-16065-3
- Carson, Jon. eBook: Genome Editing of MSCs. 2021; Available from: https://info.roosterbio.com/ebook-genome-editing-of-mscs.
- Wang, M., et al., Prediction of adeno-associated virus neutralizing antibody activity for clinical application. Gene Ther, 2015. 22(12): p. 984-92. 10.1038/gt.2015.69
- Hayashita-Kinoh, H. and T. Okada, Use of Mesenchymal Stem Cells to Enhance the Efficacy of Gene Therapy. Methods Mol Biol, 2023. 2587: p. 377-386. 10.1007/978-1-0716-2772-3_19
- Maguire, C. A., et al., Microvesicle-associated AAV vector as a novel gene delivery system. Mol Ther, 2012. 20(5): p. 960-71. 10.1038/mt.2011.303
- Brown, N., et al., Adeno-Associated Virus Vectors and Stem Cells: Friends or Foes? Hum Gene Ther, 2017. 28(6): p. 450-463. 10.1089/hum.2017.038
- Zubkova, E. S., et al., Transduction of rat and human adipose-tissue derived mesenchymal stromal cells by adeno-associated viral vector serotype DJ. Biol Open, 2021. 10(9). 10.1242/bio.058461
- Lakhan, R., et al., Local administration of AAV-DJ pseudoserotype expressing COX2 provided early onset of transgene expression and promoted bone fracture healing in mice. Gene Ther, 2015. 22(9): p. 721-8. 10.1038/gt.2015.40
