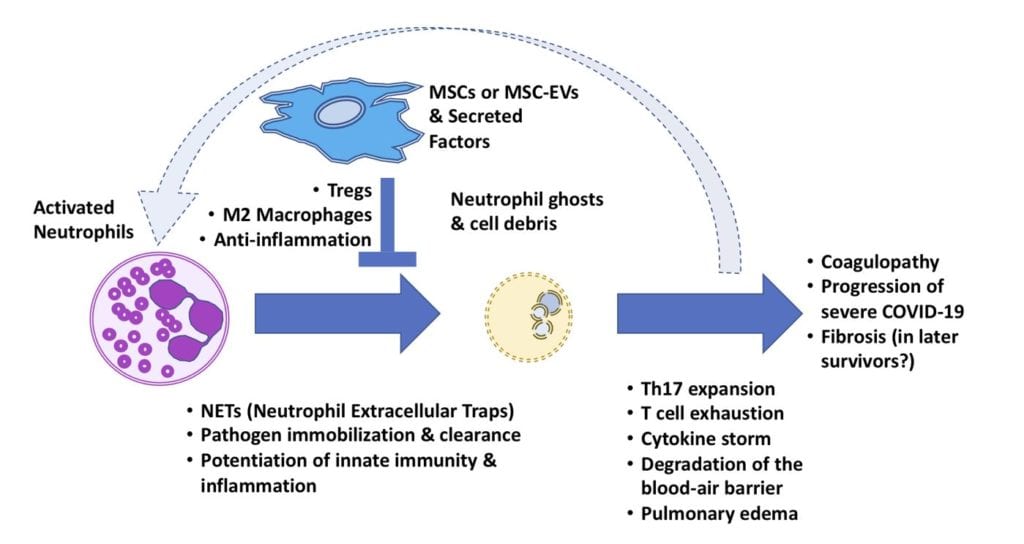
 Above, diagram of the hypothesized role of MSCs to disrupt the cycle of neutrophil “NETosis” in COVID-19 lung damage, via its immunomodulatory paracrine activity on Tregs and macrophages.
Above, diagram of the hypothesized role of MSCs to disrupt the cycle of neutrophil “NETosis” in COVID-19 lung damage, via its immunomodulatory paracrine activity on Tregs and macrophages.
Could human Mesenchymal Stromal/Stem Cells (hMSCs) break a vicious cycle seen in some COVID-19 patients? NETs from neutrophils may help as first responders near the “blaze” of infection, but perhaps therapeutic hMSCs could one day arrive as the professional “HAZMAT team” to detoxify the hot zone, saving the lungs.
Recent observations presented at the virtual annual ISCT 2020 Meeting and elsewhere [1] [2] show that we still have much to learn about COVID-19. It’s a dauntingly complex and multiphasic disease, particularly with respect to the immune response. [3] Apparent elevated risk of severe disease in cancer patients who had been treated with either immune suppression (i.e., corticosteroids) or immune stimulation (i.e., immune checkpoint inhibitor mAbs) gives anyone cause for justifiable bewilderment. When faced with paradoxes such as these, it’s time to look for new answers that might address the root of a complex pathology like COVID-19’s, one that involves non-linear feedback between multiple dynamic disease modulators. Fortunately, some clues from the anatomical “disaster scene” are beginning to emerge from behind the smoke.
De Biasi, et al (2020) recently published a detailed Nature Communications manuscript that points a firm finger at the kinetics of COVID-19’s cytokine storm and skewing of helper T lymphocytes toward a Th17 polarization. [4] Although perhaps evolutionarily adapted for anti-fungal defenses, [5] Th17 is better known for pathogenicity, related to inflammatory biomarkers like IL-17, IL-22 and IL-6. Th17 involvement is also seen in type I diabetes, multiple sclerosis, rheumatoid arthritis, and inflammatory bowel disease. However, context and milieu does matter. Some Th17-like cells can also strike a slightly contrasting chord with a “note” of cytokine expression such as IL-10’s, this time to moderate inflammatory diseases. [6] While SARS-Co-V2 infection presents with mild or moderate symptoms in most, in the smaller fraction of severe patients perhaps we recognize an immune counter-attack that is not necessarily too “mild” or too “strong,” but rather “out of tune.” The University of Modena’s observation of pathogenic Th17 and immune exhaustion in a their set of COVID-19 patients is significant, since there do exist current drugs that may specifically quiet down both Th17 mediators [7] [8] [9] and the cytokine storm (e.g., Tocilizumab). [10]
An out-of-tune and non-stop “fire alarm” with major hallmarks of Th17 can be caused by excess neutrophil activity near the infected lung lesions around the ACE2+ type II alveolar cells. Neutrophils do serve an essential purpose, as the most numerous white blood cell–and as key first responders to engulf infectious pathogens. However, they may also release “NETs” in a kind of mass suicide. Neutrophil Extracellular Traps (short video HERE) are composed of spiderweb-like chromatin and cytosolic material which immobilize pathogens and concentrate native antimicrobial factors. [11] [12]. “NETosis” can self-perpetuate via a loop involving damage- and pattern-sensing receptors (e.g., TLRs) and expelled molecular debris that resemble bacterial infection (e.g., mitochondria). If NETosis continues unabated in the human body, the result may be toxic shock, edema, organ failure and death. [13] The excess damage caused by this process could destroy organ basement membrane and microvasculature barrier function, feeding into the complement pathway, leading to coagulopathy. [14] [15] In COVID-19, blood clots and strokes are a severe problem in hospitalized patients, and they tend to strike suddenly. [16] Various reports strongly implicate neutrophils and their post-NETosis cytoplast “ghosts” as key factors in pathogenic Th17 immune system skewing. [17] [18]
Perhaps the most severe cases of COVID-19 are examples of where, first, the nominal mechanisms of the adaptive immune system failed to clear the SARS-Co-V2 viremia; and second, where the damage of the viremia to the capillary-rich lungs triggered an over-zealous innate immune response? Thus, there are at least two phases of COVID where very distinct kinds of intervention might be justified. Early in the infection, it may be helpful to assist the body’s direct fight with the infection. Later in the infection, it may be beneficial to help keep the body from destroying itself in the chaos of NETs and cytokine storm. The question of exactly when to intervene against the incipient cytokine storm remains to be resolved. The candidate therapies to use in this latter stage are becoming much clearer, however.
The use of putative Th17-quelling pharmacology may be one method with promise against COVID-19/ARDS. Nevertheless, can we ignore another potent class of immunomodulatory therapy that improves the ratio of Tregs to Th17 lymphocytes? [19] An MSC-based modality is now nearing FDA approval for a non-COVID-19 indication (i.e., Mesoblast’s remestemcel-L for GvHD), while variations of this approach are being quickly re-pivoted towards COVID-19. [20] Immunomodulation may occur in part due to the MSC secretome, [21] which can program pro-inflammatory macrophages into an anti-inflammatory or “M2” polarized phenotype, as well as induce secretion of anti-microbial peptides. [22] [23] [24] [25]
MSCs don’t merely broadcast amplifiable directions to re-educate its comrades-in-arms of the immune system. The MSC secretome can also rapidly restore lung barrier function and/or raise blood oxygen levels in ex vivo human lung [25] or in large animal models of ARDS. Hence, they can also be considered a regenerative therapy in addition to an immune-modulator. [26] In humans, results from the first COVID-19 trials with MSCs or MSC-EVs would seem to suggest a preliminary signal of clinical benefit, [27] although additional high-powered studies will be needed to identify, replicate and optimize the key parameters to determine degree of efficacy and ascertain significance. [28] It is foreseeable that new genetic engineering [29] or gene editing [30] of MSCs will super-charge payload-customized or ligand switch-controllable “living therapies” [31] [32] [33] that could be fine-tuned to better respond to the dynamic course of a COVID-19 infection in “real time.”
Wherever they are to be applied (whether for COVID-19 or other), MSCs will need to be manufactured from the highest quality starting material (qualified donor cell banks) and ancillary material (cGMP media). Furthermore, from research on to commercial scale-up, the cellular ingredients and their identity, safety, purity, potency must be comparable among manufacturing runs [34] [35] to avoid costly failures down the road. [36] [37] Therefore, in soberly reviewing lessons from the last decade of AAV and CAR-T breakthroughs (along with their “challenges”), it’s plainly obvious that a complex disease such as COVID-19 will demand support from new process innovations for the armamentarium to defeat it. These improvements will be imperative to launch new cellular therapies that are both standardized and cost-effective, [38] and not subject to the volatility of an artisanal-oriented supply chain. [39] [40] Fortunately, as we have noted before on this blog, [41] the hard work to finally render into an industrialized cellular medicine is progressing—and we believe that in the end, COVID-19 will not stand a chance.
References
- Robilotti, Elizabeth V, et al., Determinants of severity in cancer patients with COVID-19 illness. medRxiv, 2020. 10.1101/2020.05.04.20086322
- Tay, M. Z., et al., The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol, 2020. 20(6): p. 363-374. 10.1038/s41577-020-0311-8
- Akbar, A. N. and Gilroy, D. W., Aging immunity may exacerbate COVID-19. Science, 2020. 369(6501): p. 256-257. 10.1126/science.abb0762
- De Biasi, S., et al., Marked T cell activation, senescence, exhaustion and skewing towards TH17 in patients with COVID-19 pneumonia. Nat Commun, 2020. 11(1): p. 3434. 10.1038/s41467-020-17292-4
- Vautier, S., Sousa Mda, G., and Brown, G. D., C-type lectins, fungi and Th17 responses. Cytokine Growth Factor Rev, 2010. 21(6): p. 405-12. 10.1016/j.cytogfr.2010.10.001
- Wu, X., Tian, J., and Wang, S., Insight Into Non-Pathogenic Th17 Cells in Autoimmune Diseases. Front Immunol, 2018. 9: p. 1112. 10.3389/fimmu.2018.01112
- Wu, D. and Yang, X. O., TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect, 2020. 53(3): p. 368-370. 10.1016/j.jmii.2020.03.005
- Hotez, P. J., Bottazzi, M. E., and Corry, D. B., The potential role of Th17 immune responses in coronavirus immunopathology and vaccine-induced immune enhancement. Microbes Infect, 2020. 22(4-5): p. 165-167. 10.1016/j.micinf.2020.04.005
- Patruno, C., et al., Dupilumab and COVID-19: What should we expect? Dermatol Ther, 2020: p. e13502. 10.1111/dth.13502
- Campochiaro, C., et al., Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med, 2020. 76: p. 43-49. 10.1016/j.ejim.2020.05.021
- Brinkmann, V., et al., Neutrophil extracellular traps kill bacteria. Science, 2004. 303(5663): p. 1532-5. 10.1126/science.1092385
- Papayannopoulos, V., Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol, 2018. 18(2): p. 134-147. 10.1038/nri.2017.105
- Grommes, J. and Soehnlein, O., Contribution of neutrophils to acute lung injury. Mol Med, 2011. 17(3-4): p. 293-307. 10.2119/molmed.2010.00138
- Wang, Y., et al., Neutrophil extracellular trap-microparticle complexes enhance thrombin generation via the intrinsic pathway of coagulation in mice. Sci Rep, 2018. 8(1): p. 4020. 10.1038/s41598-018-22156-5
- McDonald, B., et al., Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood, 2017. 129(10): p. 1357-1367. 10.1182/blood-2016-09-741298
- Becker, R. C., COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis, 2020. 50(1): p. 54-67. 10.1007/s11239-020-02134-3
- Barnes, B. J., et al., Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med, 2020. 217(6). 10.1084/jem.20200652
- Krishnamoorthy, N., et al., Neutrophil cytoplasts induce TH17 differentiation and skew inflammation toward neutrophilia in severe asthma. Sci Immunol, 2018. 3(26). 10.1126/sciimmunol.aao4747
- Rogers, C. J., et al., Rationale for the clinical use of adipose-derived mesenchymal stem cells for COVID-19 patients. J Transl Med, 2020. 18(1): p. 203. 10.1186/s12967-020-02380-2
- Lim, M. The Race to Beat COVID-19 with hMSCs Revs Up. RoosterBio Blog 2020; Available from: https://www.roosterbio.com/covid-19/the-race-to-beat-covid-19-with-hmscs-revs-up/.
- Lim, M, Carson, J. hMSCs: A Secret(ome) Weapon Against ARDS. RoosterBio Blog 2020; Available from: https://www.roosterbio.com/covid-19/hmscs-a-secretome-weapon-against-ards/.
- Krasnodembskaya, A., et al., Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells, 2010. 28(12): p. 2229-38. 10.1002/stem.544
- Jackson, M. V., et al., Mitochondrial Transfer via Tunneling Nanotubes is an Important Mechanism by Which Mesenchymal Stem Cells Enhance Macrophage Phagocytosis in the In Vitro and In Vivo Models of ARDS. Stem Cells, 2016. 34(8): p. 2210-23. 10.1002/stem.2372
- Matthay, M. A., Pati, S., and Lee, J. W., Concise Review: Mesenchymal Stem (Stromal) Cells: Biology and Preclinical Evidence for Therapeutic Potential for Organ Dysfunction Following Trauma or Sepsis. Stem Cells, 2017. 35(2): p. 316-324. 10.1002/stem.2551
- Park, J., et al., Therapeutic effects of human mesenchymal stem cell microvesicles in an ex vivo perfused human lung injured with severe E. coli pneumonia. Thorax, 2019. 74(1): p. 43-50. 10.1136/thoraxjnl-2018-211576
- Asmussen, S., et al., Human mesenchymal stem cells reduce the severity of acute lung injury in a sheep model of bacterial pneumonia. Thorax, 2014. 69(9): p. 819-25. 10.1136/thoraxjnl-2013-204980
- Leng, Z., et al., Transplantation of ACE2(-) Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis, 2020. 11(2): p. 216-228. 10.14336/AD.2020.0228
- Qu, W., et al., Cell-based therapy to reduce mortality from COVID-19: Systematic review and meta-analysis of human studies on acute respiratory distress syndrome. Stem Cells Transl Med, 2020. 10.1002/sctm.20-0146
- Lee, P., et al., Manufacturing Development of SENTI-101, a Gene Circuit Modified Allogeneic Bone Marrow Derived Mesenchymal Stromal Cell (BM-MSC) Therapy for the Treatment of Solid Tumors. Cytotherapy, 2020. 22(5, Supplement): p. S11-S12. https://doi.org/10.1016/j.jcyt.2020.03.474
- Srifa, W., et al., Cas9-AAV6-engineered human mesenchymal stromal cells improved cutaneous wound healing in diabetic mice. Nat Commun, 2020. 11(1): p. 2470. 10.1038/s41467-020-16065-3
- Huang, C., et al., Therapeutic effect of intratumoral administration of DCs with conditional expression of combination of different cytokines. Cancer Immunol Immunother, 2012. 61(4): p. 573-9. 10.1007/s00262-011-1198-9
- Kojima, R., Scheller, L., and Fussenegger, M., Nonimmune cells equipped with T-cell-receptor-like signaling for cancer cell ablation. Nat Chem Biol, 2018. 14(1): p. 42-49. 10.1038/nchembio.2498
- Scheller, L., et al., Phosphoregulated orthogonal signal transduction in mammalian cells. Nat Commun, 2020. 11(1): p. 3085. 10.1038/s41467-020-16895-1
- Christy, Barbara A, et al. Mesenchymal Stem Cells Grown in a Bioreactor Are Functionally Similar to Those Grown in Monolayer Culture. in 2019 Annual Meeting. 2019. AABB.
- Kirian, RD, et al., SCALING A XENO-FREE FED-BATCH MICROCARRIER SUSPENSION BIOREACTOR SYSTEM FROM DEVELOPMENT TO PRODUCTION SCALE FOR MANUFACTURING XF hMSCs. Cytotherapy, 2019. 21(5): p. S71-S72.
- Witcher, Mark. Phase III Clinical Trials – Ever Wonder Why Some Products Unexpectedly Fail? Pharmaceutical Engineering 2019; Available from: https://ispe.org/pharmaceutical-engineering/ispeak/phase-iii-clinical-trials-ever-wonder-why-some-products-unexpectedly-fail.
- Witcher, Mark. Why Controlling CQAs Isn’t Good Enough For Gene & Cell Therapies. Cell & Gene 2020; Available from: https://bit.ly/2xBvCHj.
- Galipeau, J. and Sensebe, L., Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell, 2018. 22(6): p. 824-833. 10.1016/j.stem.2018.05.004
- Rowley, Jon, et al., Meeting lot-size challenges of manufacturing adherent cells for therapy. BioProcess Int, 2012. 10(3): p. 7.
- Rowley, Jon A and Montgomery, SA, The need for adherent cell manufacturing: production platform and media strategies drive cell production economics. BioProcess Int, 2018. 16: p. 34-49.
- Carson, J., Farrance, I., Getz, J., and Jon A. Rowley. Scalable MSC Manufacturing Matters in a Rapid Response to COVID-19. RoosterBio Blog 2020; Available from: https://www.roosterbio.com/biomanufacturing/scalable-msc-manufacturing-matters-in-a-rapid-response-to-covid-19/.
