
Section of an Oil Painting, from Paul Gaugiun’s Where Do We Come From? What Are We? Where Are We Going? (1897), Full Painting.
Start With the Basics
Whether you’re a post-impressionist artist or the mastermind of extracellular vesicle/exosome (EV) therapeutics, it’s both confounding and useful to launch a project based on the three most essential questions. Why “confounding?” Well, a thinking person’s answer is usually “it depends,” since additional hard work is required to penetrate deeper meaning. However, this query is “useful” because it orients the exosome product based on a definition (“What are we?”) [1, 2, 3, 4, 5] in the full light of its process (“Where do we come from?”) [6, 7, 8, 9, 10, 11] and ultimate use (“Where are we going?”). [11, 12, 13, 14, 15] In October of 2022, Trey Picou, Ph.D., and Stephen Lenzini, Ph.D. broadcast a well-received webinar on EV Analytics to help remove some of the weight off of these heavy extracellular vesicle product development questions. Their key answer lies in the analytics [16, 17] that address how an exosome product’s process aligns with its intended identity. This blog summarizes their highly recommended lecture.
Let’s first go over the “Final Takeaways” and then dive into some of the supporting insights:
|
|
|
Drs. Lenzini and Picou provide you with ample supporting examples and rationale to justify these high-level insights in a tight, 43-minute presentation.
What Are the Important Parameters?
Dr. Stephen Lenzini names characteristics and properties that define an extracellular vesicle according to the latest investigation and the guidelines of MISEV (Minimal Information for Studies of Extracellular Vesicles). MISEV guidelines [2, 18] were established to improve the quality of peer-reviewed extracellular vesicle/exosome studies such that the correct analytic methods could be brought to bear on solid conclusions that advance the state of knowledge. This initiative is especially important, given that extracellular vesicles derived from MSCs are the basis for a ground swelling in new and targetable molecular therapeutics, with several dozen now entering human trials. The MISEV community recommends that extracellular vesicles are quantitatively characterized at the bulk (population) level by source parameters (media, cells, tissue mass, population doubling), particle concentration and size distribution, total content of macromolecules (protein, lipid, nucleic acid), and surface markers. In addition, extracellular vesicles could be characterized at the single-particle level by higher throughput, lower sensitivity methods (e.g., fNTA and nano-flow cytometry) and/or lower-throughput, higher sensitivity methods like electron microscopy, atomic force microscopy, and SP-IRIS. Of recent interest are techniques currently in development, including super-resolution microscopy and single-particle automated Raman trapping analysis (SPARTA).
Understand the Process
One common descriptor applied to extracellular vesicles/exosomes is “heterogeneous;” the one similarity shared by all extracellular vesicle populations is that they all can be different in subtle ways! Hence, the original extracellular vesicle source material (e.g., cell culture, milk, in vivo fluids), the upstream process, and downstream isolation methods all can dramatically affect each of these MISEV recommended parameters. The upstream process can be optimized by improvements in each step from the seed train to the point of extracellular vesicle collection. It’s useful to keep an eye on extracellular vesicle productivity here—that is, the total # cells x extracellular vesicles/cell. In our webinar, Lenzini details exactly how to go about this.
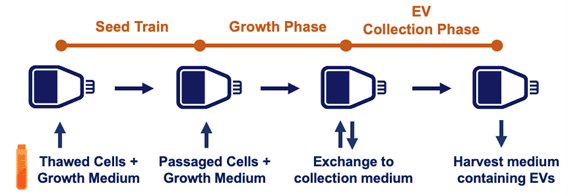
Above, a schematic of a generic extracellular vesicle “upstream” process.
Next, Lenzini considers downstream processing (DSP) of an extracellular vesicle product, which involves harvest, clarification, concentration, purification, and formulation. During DSP steps, he notes that analytical methods will address questions like how each step affects extracellular vesicle recovery, whether extracellular vesicle identity is maintained or how their macromolecular cargoes change, and if the extracellular vesicle processing ultimately preserves biological function in units of potency. If developing extracellular vesicle material into a drug, MISEV-inspired analytics can directly tie into the material’s target product profile (TPP) and the critical quality attributes (CQAs) that aim to consistently define it. Therefore, Lenzini concludes, “During development, it benefits to pay constant attention to extracellular vesicle CQAs utilizing current and best analytical practices.”
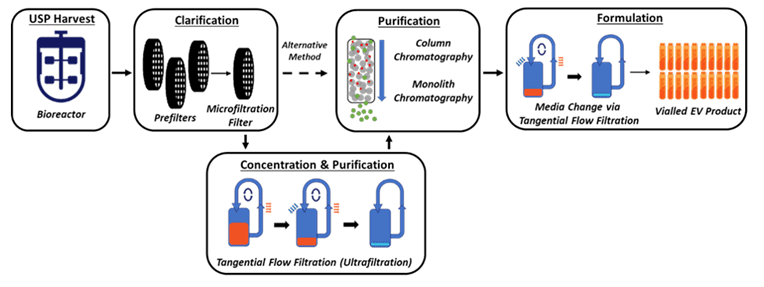
Above, schematic of a generic extracellular vesicle “downstream” process.
Process- & Product-Appropriate Analytical Methods
After Dr. Stephen Lenzini’s remarks, Dr. Trey Picou gives a perspective on the important analytics related to the extracellular vesicle product as rigorously defined by its emergent features from a dynamic and complex process. But what’s essential and what amounts to splitting hairs? Realistically, a one-size-fits-all analytical method does not exist; the developer must choose methods that provide insights into one’s own unique CQAs. Broadly speaking, Picou says that complementary modalities are often needed to understand what’s going on, i.e., bulk analytics and individual analytics. Bulk analytics will answer if the source material is consistent, assessing concentration and size distribution of the extracellular vesicle population, and will ask if a measurable process parameter can be found in a sample from a given process step. Complementary individual analytics could help answer whether specific extracellular vesicle particle types within the population address a critical parameter.
Picou discusses some examples of highly useful instrumentation and its role in RoosterBio’s analytical services for extracellular vesicles. There’s the ZetaView® x30 QUATT from Particle Metrix, which provides extracellular vesicle concentration and size distribution measurements. [19] By using a specialized dye (MemGlow) combined with the instrument’s fluorescent detection capabilities, extracellular vesicles comprised of a lipid bilayer can be distinguished from other particles lacking a lipid bilayer. Detecting surface markers involves Western blotting for presence of intact extracellular vesicle-specific tetraspanin surface markers (CD63, CD9, CD81). Such markers can also be quantitatively measured, of course, with parallel ELISAs. Similarly, extracellular vesicle contents are routinely dissected with standard bulk measurements of total protein (microBCA), lipid (MemGlow), and small RNAs (quantitative gel or capillary electrophoresis). Furthermore, a state-of-the-art nanoFCM (nano-flow cytometry) instrument can gather rapid, simultaneous, multi-modal readouts from extracellular vesicle populations to assess size, concentration, or multiple surface markers,—or artificially engineered targeting handles. [20] With this instrument’s powerful capability to measure a population’s particle side scatter, green fluorescence (FITC), or red fluorescence (PE), an assay development effort via the nanoFCM can establish a routine detection system for many extracellular vesicle marker-selective probes with its 488nm CW excitation laser and correctly gated photodetectors.
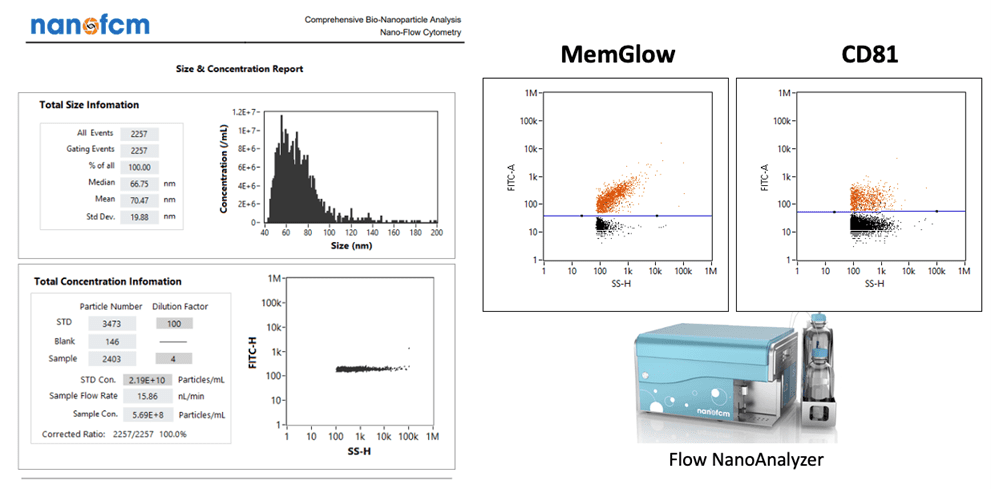
Above, an example of multi-modal readouts of extracellular vesicle particle analyses via the nanoFCM instrument system
To assess potency and extracellular vesicle’s clinically relevant biofunction, assays can be tailored directly toward an intended therapeutic mechanism. Extracellular vesicles are sometimes considered for wound healing indications, where dose responsiveness can be gauged with “scratch assays” that employ cell image quant software to track the reclosure of a broken lawn of HUVEC or primary fibroblast cells. Similarly, extracellular vesicles can augment wound healing through accelerated angiogenesis. Picou’s company, RoosterBio, is similarly well-versed in angiogenesis assays to digitally capture the effect of extracellular vesicles on HUVEC tube formation lengths in Matrigel. However, there may be numerous other bioactivities of interest related to a product developer’s extracellular vesicles, such as immune modulation or targeted cancer cell deletion. Dr. Picou says that the harvest of these unique measurements can be readily adapted via custom assay development services. This custom work would then enable future editions of more expansive assay “catalogs.”
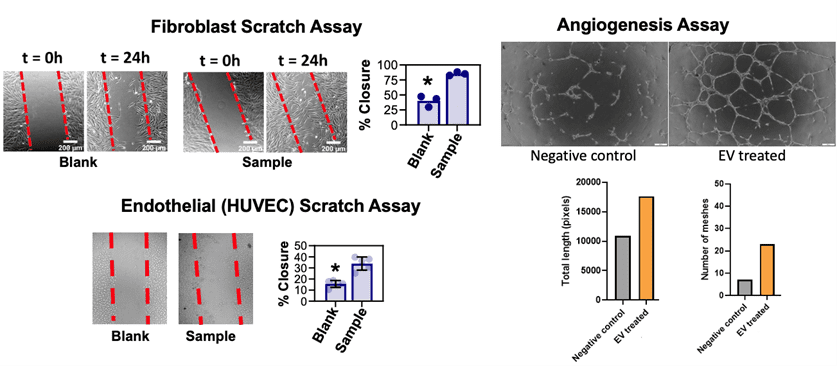
Above, examples of image-quantified biological potency readouts of EV/exosome bioactivity
Work With a Partner Who Understands the Complexities
As an extracellular vesicle/exosome clinical product developer, you might wish to leverage a “division of labor” for big gains in time efficiency as you hustle in the race toward clinical trials. Dr. Trey Picou says that a flexible partner is most preferred—one with a team who deeply understands that there isn’t a “one size fits all” for every extracellular vesicle/exosome process solution. That is, your very own company will undoubtedly house the leading experts to define a most optimal Target Product Profile (TPP) to combat your disease indication of choice. Further, your team would understand the more complex in vivo assays and tox studies for IND enabling, as well as the finer points of trial design and patient cohort stratification. On the other hand, a competent partner with bandwidth and capacity ought to first provide you with an instant menu of routine EV analytical services, capable of handling and qualifying samples taken from production cells (“upstream”) all way to the very end—a vialed “downstream” product. A quick reply with the scope, cost, and reasonable turnaround time of these standard activities would also speak to the kind of flexibility and diligence that could be mustered for any add-on custom development services. Working collaboratively, your team and a decent EV analytics crew-for-hire thus could reach your objectives much more quickly—and with fewer hassles.
Dr. Picou also names some analytical services of likely high relevance to your extracellular vesicle-producing cells. These might include cell expansion analysis, surface marker characterization, differentiation or immunomodulatory potential, angiogenic secretome analysis, and karyotyping. As Picou recaps from earlier in the lecture, basic extracellular vesicle/exosome analytics would include metrics capturing particle size and concentration (# particles, protein amount), purity (e.g., % lipidated, % RNA subtypes), identity (surface markers and specific miRNA or probe cargoes), or potency. In most cases, such menu items do not just come from nowhere. — Like the proverbial “tip of the iceberg,” these productized services likely speak from a solid track record of prior custom projects that may range from extracellular vesicle upstream and downstream process development to bioprocess scale-up, cell line engineering, and work with non-human exosomes (e.g., cow’s milk).
Picou concludes the webinar by noting his exemplary extracellular vesicle analytics service team at RoosterBio as one that characterizes each step of the upstream process, downstream process, and the assay development to support its analytics—from start to finish. Upstream characterization involves both RUO as well as cGMP grade human mesenchymal stem cells (hMSCs) that are used in seed trains for reliable expansion into 3D bioreactors. Downstream process characterization involves RoosterBio’s growing expertise in extracellular vesicle separation, purification, and fill/finish. Finally, RoosterBio makes sure these extracellular vesicle or cellular products fit safety guidelines and the original product CQAs. For RoosterBio product and service customers, its processes are specifically designed to deliver consistent population doublings at all scales—from bench-top pilot projects via 2D planar cellular to clinically relevant bioreactor runs with over 100 billion cells or 1 x 1015 EVs.
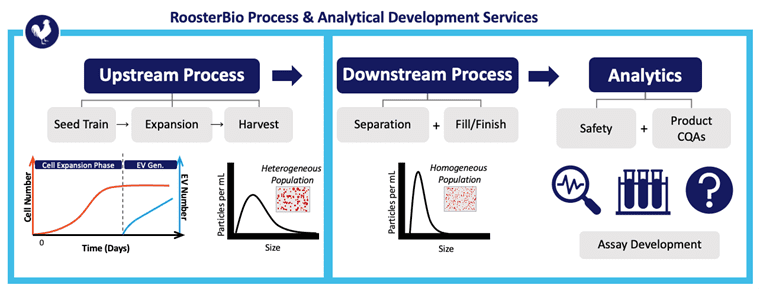
Above, RoosterBio is an exemplar for standard and custom analytical services to assist in extracellular vesicle/exosome upstream process development, DSP, and analytics of the vialed product.
After concluding, this webinar EV Analytics: Know Your Process & Define Your Product, delivered by Stephen Lenzini, Ph.D., and Trey Picou, Ph.D., generated many audience questions. If you would also like to learn more, we invite you to watch the webinar and likewise ask us your questions about what you’ve viewed and how we can help you find your way around the challenges of extracellular vesicles/exosomes and their related analytics.
References
- Witwer, K. W., et al., Updating the MISEV minimal requirements for extracellular vesicle studies: building bridges to reproducibility. J Extracell Vesicles, 2017. 6(1): p. 1396823. 10.1080/20013078.2017.1396823
- Thery, C., et al., Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles, 2018. 7(1): p. 1535750. 10.1080/20013078.2018.1535750
- Witwer, K. W. and C. Thery, Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J Extracell Vesicles, 2019. 8(1): p. 1648167. 10.1080/20013078.2019.1648167
- Kugeratski, F. G., et al., Quantitative proteomics identifies the core proteome of exosomes with syntenin-1 as the highest abundant protein and a putative universal biomarker. Nat Cell Biol, 2021. 23(6): p. 631-641. 10.1038/s41556-021-00693-y
- Pathan, M., et al., Vesiclepedia 2019: a compendium of RNA, proteins, lipids and metabolites in extracellular vesicles. Nucleic Acids Res, 2019. 47(D1): p. D516-D519. 10.1093/nar/gky1029
- Mathieu, M., et al., Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat Commun, 2021. 12(1): p. 4389. 10.1038/s41467-021-24384-2
- Ng, K. S., et al., Bioprocess decision support tool for scalable manufacture of extracellular vesicles. Biotechnol Bioeng, 2019. 116(2): p. 307-319. 10.1002/bit.26809
- Lenzini, S., et al., Exosomes/EVs: DEVELOPING A MICROCARRIER STIRRED TANK PROCESS FOR LARGE-SCALE HMSC-EV PRODUCTION. Cytotherapy, 2022. 24(5): p. S91.
- Lenzini, Stephen. Extracellular Vesicle/Exosome Upstream Process Development: Maximizing Productivity to Accelerate Clinical Adoption. 2022; Available from: https://www.roosterbio.com/blog/extracellular-vesicle-exosome-upstream-process-development-maximizing-productivity-to-accelerate-clinical-adoption/.
- Jung, Jai and Lenzini, Stephen. Extracellular Vesicle/Exosome Downstream Process Development Part I: Leveraging Filtration Technologies for Scalable EV Preparation. 2022; Available from: https://www.roosterbio.com/blog/extracellular-vesicle-exosome-downstream-process-development-part-i-leveraging-filtration-technologies-for-scalable-ev-preparation/.
- Lenzini, Stephen. Establishing a Working Range for Effective MSC-EV Dose. RoosterBio Blog 2021; Available from: https://www.roosterbio.com/blog/establishing-a-working-range-for-effective-msc-ev-dose/.
- Lener, T., et al., Applying extracellular vesicles based therapeutics in clinical trials – an ISEV position paper. J Extracell Vesicles, 2015. 4: p. 30087. 10.3402/jev.v4.30087
- Borger, V., et al., International Society for Extracellular Vesicles and International Society for Cell and Gene Therapy statement on extracellular vesicles from mesenchymal stromal cells and other cells: considerations for potential therapeutic agents to suppress coronavirus disease-19. Cytotherapy, 2020. 22(9): p. 482-485. 10.1016/j.jcyt.2020.05.002
- Lenzini, Stephen. Big Effects in Small Packages: What Are Extracellular Vesicles, Exosomes, & Microvesicles & Why Are They En Route to the Clinic? 2021; Available from: https://www.roosterbio.com/blog/big-effects-in-small-packages-what-are-extracellular-vesicles-exosomes-microvesicles-why-are-they-en-route-to-the-clinic/.
- Lui, P. P. Y. and Y. T. Leung, Practical Considerations for Translating Mesenchymal Stromal Cell-Derived Extracellular Vesicles from Bench to Bed. Pharmaceutics, 2022. 14(8). 10.3390/pharmaceutics14081684
- Van Deun, J., A. Hendrix, and Ev-Track consortium, Is your article EV-TRACKed? J Extracell Vesicles, 2017. 6(1): p. 1379835. 10.1080/20013078.2017.1379835
- Coumans, F. A. W., et al., Methodological Guidelines to Study Extracellular Vesicles. Circ Res, 2017. 120(10): p. 1632-1648. 10.1161/CIRCRESAHA.117.309417
- Lotvall, J., et al., Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles, 2014. 3: p. 26913. 10.3402/jev.v3.26913
- Bachurski, D., et al., Extracellular vesicle measurements with nanoparticle tracking analysis – An accuracy and repeatability comparison between NanoSight NS300 and ZetaView. J Extracell Vesicles, 2019. 8(1): p. 1596016. 10.1080/20013078.2019.1596016
- Tian, Y., et al., Quality and efficiency assessment of six extracellular vesicle isolation methods by nano-flow cytometry. J Extracell Vesicles, 2020. 9(1): p. 1697028. 10.1080/20013078.2019.1697028
