“Every jumbled pile of person has a thinking part that wonders
What the part that isn’t thinking isn’t thinking of”Where Your Eyes Don’t Go
-They Might Be Giants (1988) [1]
When you’re zeroed in on one thing, you’re also not focused on…well…everything else. Then your shoulder’s “imp of distraction” digs in his claws, dancing on your hard-won focus, asking if there aren’t more valuable things to scan on the horizon. As sung in this zany quote from the TMBG catalog, where to best fixate our fallible attention tops life’s most humbling vexations! [2] Perhaps nowhere is this dilemma more palpable than when sifting through regenmed clinical trial data. [3] Yet in this article, we’re happy to report the fortunate results of one wider glance. Regardless of your favorite nomenclature for adipose tissue-derived hMSCs (hAD-MSCs, [4] MSC(AT), [5] AT-MSCs [6]), there really is more beneath their story than meets the eye. The instinct behind this attention has driven RoosterBio’s work with them since before 2015. [4] Want to learn more? [7] Stay tuned! Some interesting developments are about to reach fruition.
Take a look at this chart (below; data charted from celltrials.org reporting), based on the subset of hAD-MSC data from celltrials.org. The count of new posted clinical trials in the world’s major databases trends for a solid year-to-year increase between 2011 and 2021.
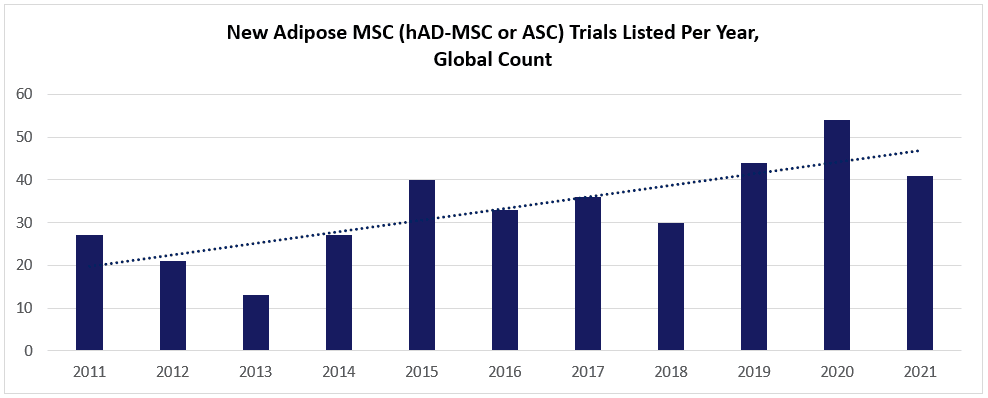
Viewed superficially, it’s tempting to glibly assume that these trials heavily favor autologous-administered hAD-MSCs over allogeneic. As we and others have noted, adipose-derived MSCs (aka “Adipose Stem Cells” or “ASCs”) do provide an abundant source of cellular material that’s much easier to extract than from bone marrow, and, also easier to isolate a homogeneous population than from umbilical cords. [8] At a patient’s bedside, one could realistically get 1+ liters from the lipoplasty, isolate the cells via the tissue’s stromal vascular fraction (SVF), and then re-implant a dose of 100s of millions of hAD-MSCs. Using automated closed system instruments like Cytori’s Celution®, such could be envisaged to occur in just one day’s hospital visit. The potential ease of access to hAD-MSC material thus beckons a question: If getting a minimum cell dose does not constrain autologous hAD-MSCs because of the high-volume sample, why risk new challenges related to allogeneic donor sourcing, banking, and quality control?
Lo and behold, the last decade shows us a trend that bucks the conventional wisdom. When we segment the numbers of annual hAD-MSC trials into autologous and allogeneic groups, there’s a surprise. Beginning around 2015, hAD-MSCs are much less monopolized by autologous uses. As shown in the chart (below; data charted from celltrials.org reporting), we see that clinical developers increasingly opt for allogeneic hAD-MSCs into the 30-50% range. Arguably, much of this hAD-MSC growth across the decade can be attributed to allogeneic applications.
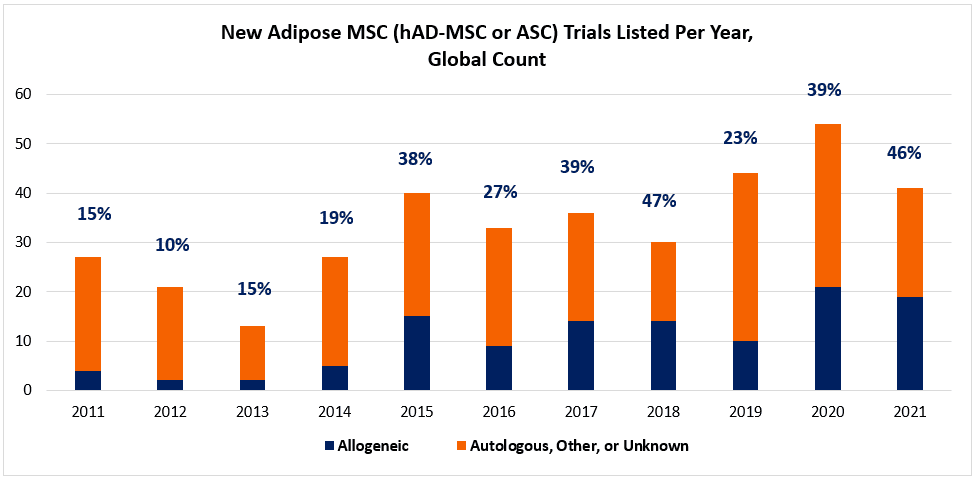
As usual, digging a little more deeply into a mystery is wont to yield more questions than answers. A reasonable place to start is “Why?” If autologous was really so much more convenient, why are many turning to “allo-“ sources of hAD-MSCs? Does a clue lie in the classes of disease indications that might be preferred by allo-hAD-MSCs vs. autologous hAD-MSCs?
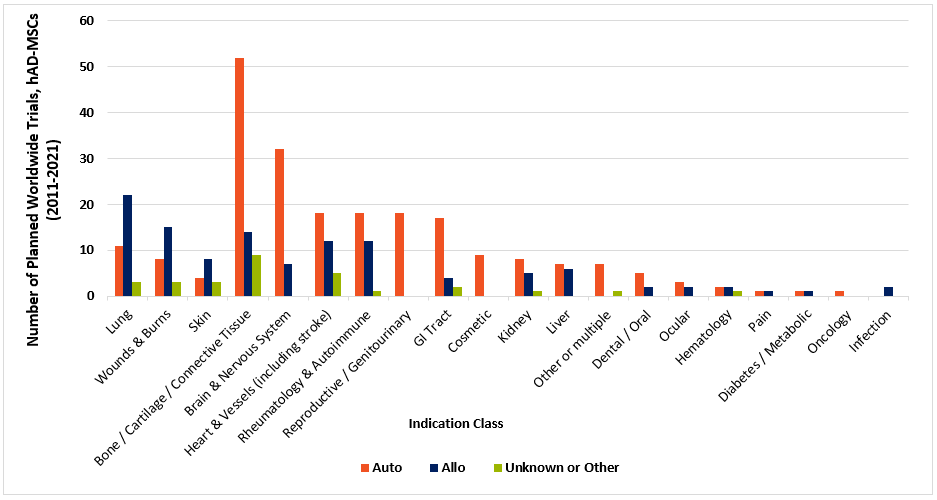
As depicted in the chart (above; data charted from celltrials.org reporting) of hAD-MSC related clinical trials between 2011 and 2021, autologous applied cells still have the upper hand for most classes of indications.
Nevertheless, allogeneic uses outnumber autologous in diseases of the lung, wounds & burns, and skin. One reason may lie in fat tissue’s natural role in human physiology. It contributes a layer of insulation to maintain body temperature, provides a reserve bank of caloric energy, and cushions skin and organs. Further, it’s highly vascularized, allowing a circulatory route for immune cells to efficiently roll into zones of injury or infection. In serious burns and wounds, there’s likely major trauma to existing reservoirs of subcutaneous or abdominal fat. Finally, those with severe burns or wounds may be apt to suffer from emaciation and cachexia during their prolonged recovery.
Is surgically “wounding” this bank of native fat tissue a case of adding insult to injury, like “Robbing Peter to pay Paul?” In contrast, perhaps it’s better to obtain a donor transplant of reinforcement “rescue” cells while additionally drawing from one’s own body’s reserve? As for lungs, 16/22 of allo- hAD-MSC lung therapies were for COVID-19 related conditions, and 4 were for ARDS. As unfortunate experience from the last 2+ years taught us, SARS-CoV2 infection can wreak havoc quickly and unexpectedly, at times overwhelming ICUs. Acute care, managed hour-by-hour, surely can’t wait to muster the logistics and expertise for lipoplasty around a ward full of infectious, critically ill patients. But a large, nearby stockpile of hAD-MSCs could get the job done. RoosterBio has written about the ongoing need for such stockpiles in case similar emergencies threaten again. [9]
So, it looks like allogeneic hAD-MSCs (instead of autologous hAD-MSCs) might be favored by (1) those who need to quickly draw from a ready-to-use bank for acute conditions, and (2), those who want to topically or locally administer doses [10] to patients with severe injuries. At this juncture, it’s probably worth recalling why allogeneic MSCs in general could be a favorable option in comparison with autologous MSCs. [11, 12] First, allogeneic cells can derive from a fully characterized, quality-controlled source that can be cryogenically stored. Next, the desired cells can be isolated and expanded—with qualified materials—out of an industrialized supply chain to obtain a master cell bank (MCB) and its “daughter” working cell bank (WCB). In RoosterBio’s case, these cell banks can be linked to Drug Master Files (DMFs) with the FDA for filing the IND.
In contrast to “allo,” it’s intrinsically challenging to predict and validate quality cell sourcing with an autologous therapy, even if the intention is to exploit possible advantages of “fresh” [13] vs. the tradeoff of mixed patient age and health. [14] As we’ve written before, quality truly does “begin at inception.” [15] Although there clearly exist many valuable applications for autologous MSCs, there cannot be a revolution in scale and cost-reduction to democratize cell and gene therapies unless well-qualified allogeneic cells play a foundational role. Even so, we and others have written about exciting breakthrough applications where allogeneic MSCs can “tag team” with autologous cells as instrumental components of the cell therapy drug process. [16, 17, 18]
Allogeneic or not, hAD-MSCs have been growing in clinical trial presence over the last decade. We already know that adipose is a large-volume tissue that’s highly enriched in MSCs. What other features might make adipose MSCs “special?” Comparisons between multiple MSC types are not very common—and when they are, the results are not always consistent. [19, 20] Neither are the biological readouts or experimental applications uniformly harnessed. This is probably because, in addition to donor-specific variables like age and health, [21] there are also differences even within various types and layers of adipose tissue (e.g., abdominal vs. subcutaneous), and variations in how these cells were expanded into working cell banks. [22]
Caveats aside, some patterns do reign constant. Yielding to the imprint of anatomical milieu, adipose MSCs can more readily differentiate into fat on stimulation. They tend to expand through increased rounds of population doubling, and exhibit more elevated CFUs than bone marrow counterparts, perhaps due to maintenance in a more stem-like state [23] with higher expression of master regulators such as TWIST1, [24] Nanog, c-Myc [25] and rheostatic signaling via bFGF/FGF2 through MAPK/Erk.
Some fascinating work also suggests a continuum that is spread between two poles of biofunction: (1) MSC populations that are more prone to self-renewal and angiogenesis and (2) MSCs that expand less and show stronger immunomodulation. [24] While hAD-MSCs are reported to modulate both pro-angiogenic [26] and anti-inflammatory [27] effects, some findings from “omics” studies [28] may suggest that hAD-MSCs could have greater talent than bone marrow MSCs for certain therapeutic mechanisms that favor new microvasculature sprouting. However, still other studies show that hAD-MSCs might be intermediate in their pro-angiogenic activity and expansion potential, behind MSCs derived from umbilical cord. [29] Adding to the complexity, it’s quite possible that tissue origin might prove to be a lesser phenotypic governor than once thought. Instead, “outside-in” signaling from hard vs. “squishy” growth substrates and/or the repertoire of integrin and matrix attachments might be at least as defining. [23]
Is it too early to generalize hAD-MSCs as a “happy medium” type that grows slightly more than bone marrow MSCs, but less than cord MSCs? That they are less anti-inflammatory than MSCs of bone marrow origin—but better suited to this role than cord MSCs? Perhaps. Technology will certainly be a boon for all three major MSC types. It will be interesting to note ongoing new findings to suggest practical avenues for process- and indication-specific priming or genetic modification [30] of hAD-MSCs (and all their MSC “cousins”) to deterministically drive their therapeutic phenotype.
Closing this blog, it’s incidental to note that the artists with the opening quote (They Might Be Giants) have been famous for nearly as long as medicine has been fascinated with “Mesenchymal Stem Cells.” Both TMBG and the MSC field has evolved considerably since then. [31, 32] Mesenchymal Stromal Cells… Medicinal Signaling Cells… [33] MSCs… Whatever nomenclature our colleagues at ISCT wish to assign to them, [5, 34] three favorite words of TMBG fans will surely define the next decade for MSCs: “Here Comes Science!”
References
- Giants, They Might Be. Where Your Eyes Don’t Go. 1988; Available from: https://www.youtube.com/watch?v=Ro_u6AhcGYo.
- Chabris, Christopher F. and Daniel J. Simons, The invisible gorilla: And other ways our intuitions deceive us. 2010: Harmony.
- celltrials.org. Cell Trials Data. celltrials.org 2021; Available from: https://celltrials.org/cells-data
- RoosterBio. Adipose & Bone Marrow-Derived hMSCs: What’s the Difference? RoosterBio Blog 2015; Available from: https://www.roosterbio.com/blog/adipose-and-bone-marrow-derived-hmscs-whats-the-difference/.
- Viswanathan, S., et al., Consensus International Council for Commonality in Blood Banking Automation-International Society for Cell & Gene Therapy statement on standard nomenclature abbreviations for the tissue of origin of mesenchymal stromal cells. Cytotherapy, 2021. 23(12): p. 1060-1063. 10.1016/j.jcyt.2021.04.009
- Freitas, G. P., et al., Cell Therapy: Effect of Locally Injected Mesenchymal Stromal Cells Derived from Bone Marrow or Adipose Tissue on Bone Regeneration of Rat Calvarial Defects. Sci Rep, 2019. 9(1): p. 13476. 10.1038/s41598-019-50067-6
- RoosterBio. “adipose” Query to RoosterBio.com. 2014-202? ; Available from: https://bit.ly/394klkR.
- Carson, Jonathan, hUC-MSCs Are Unique: Are the Biomaterials, Bioprocesses, & Clinical Product Developers Now “in Accord”? 2021: RoosterBio Blog 2021; Available from https://www.roosterbio.com/blog/huc-mscs-are-unique-are-the-biomaterials-bioprocesses-clinical-product-developers-now-in-accord/
- Olsen, T. MSCs as Medical Stockpiled Countermeasures: Cellular “First Responders” for COVID-19 and Future Emerging Biothreats. 2020; Available from: https://www.roosterbio.com/blog/mscs-as-medical-stockpiled-countermeasures-cellular-first-responders-for-covid-19-and-future-emerging-biothreats/.
- Moll, G., et al., Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines. Trends Mol Med, 2019. 25(2): p. 149-163. 10.1016/j.molmed.2018.12.006
- Laloze, J., L. Fievet, and A. Desmouliere, Adipose-Derived Mesenchymal Stromal Cells in Regenerative Medicine: State of Play, Current Clinical Trials, and Future Prospects. Adv Wound Care (New Rochelle), 2021. 10(1): p. 24-48. 10.1089/wound.2020.1175
- Li, C., et al., Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci, 2021. 11(1): p. 187. 10.1186/s13578-021-00698-y
- Moll, G., et al., Cryopreserved or Fresh Mesenchymal Stromal Cells: Only a Matter of Taste or Key to Unleash the Full Clinical Potential of MSC Therapy? Adv Exp Med Biol, 2016. 951: p. 77-98. 10.1007/978-3-319-45457-3_7
- Phinney, D. G., Functional heterogeneity of mesenchymal stem cells: implications for cell therapy. J Cell Biochem, 2012. 113(9): p. 2806-12. 10.1002/jcb.24166
- Williams, K.; Hansen, C. . Quality Begins at Inception. 2020; Available from: https://www.roosterbio.com/blog/quality-begins-at-inception/.
- Carson, J. MSCs “Versus” or MSCs “Via” T Cells for Cell & Gene Therapy? RoosterBio Blog 2022; Available from: https://www.roosterbio.com/blog/mscs-versus-or-mscs-via-t-cells-for-cell-gene-therapy/.
- McKenna, M. K., et al., Mesenchymal stromal cell delivery of oncolytic immunotherapy improves CAR-T cell antitumor activity. Mol Ther, 2021. 29(5): p. 1808-1820. 10.1016/j.ymthe.2021.02.004
- Alvaro, D., Patel, A. Accelerating the Development of a Novel Cell Therapy for COVID-19–Associated Lung Fibrosis. 2021; Available from: https://www.pharmasalmanac.com/articles/accelerating-the-development-of-a-novel-cell-therapy-for-covid-19-associated-lung-fibrosis.
- Kern, S., et al., Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells, 2006. 24(5): p. 1294-301. 10.1634/stemcells.2005-0342
- Hass, R., et al., Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal, 2011. 9: p. 12. 10.1186/1478-811X-9-12
- Ujiie, Yuko, Kazuhiro Gomi, and John Edward Davies, MSC functional phenotype: Assay, age and source dependence. Science Proceedings, 2015. 2. 10.14800/sp.934
- Mangum, L. H., et al., Tissue Source and Cell Expansion Condition Influence Phenotypic Changes of Adipose-Derived Stem Cells. Stem Cells Int, 2017. 2017: p. 7108458. 10.1155/2017/7108458
- Pittenger, M. F., et al., Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med, 2019. 4: p. 22. 10.1038/s41536-019-0083-6
- Boregowda, S. V., et al., A Clinical Indications Prediction Scale Based on TWIST1 for Human Mesenchymal Stem Cells. EBioMedicine, 2016. 4: p. 62-73. 10.1016/j.ebiom.2015.12.020
- Hardy, W. R., et al., Transcriptional Networks in Single Perivascular Cells Sorted from Human Adipose Tissue Reveal a Hierarchy of Mesenchymal Stem Cells. Stem Cells, 2017. 35(5): p. 1273-1289. 10.1002/stem.2599
- Krawczenko, A. and A. Klimczak, Adipose Tissue-Derived Mesenchymal Stem/Stromal Cells and Their Contribution to Angiogenic Processes in Tissue Regeneration. Int J Mol Sci, 2022. 23(5). 10.3390/ijms23052425
- Leto Barone, A. A., et al., Immunomodulatory effects of adipose-derived stem cells: fact or fiction? Biomed Res Int, 2013. 2013: p. 383685. 10.1155/2013/383685
- Wang, Z., et al., Single-cell transcriptome atlas of human mesenchymal stem cells exploring cellular heterogeneity. Clin Transl Med, 2021. 11(12): p. e650. 10.1002/ctm2.650
- Pinto, D. S., et al., Modulation of the in vitro angiogenic potential of human mesenchymal stromal cells from different tissue sources. J Cell Physiol, 2020. 235(10): p. 7224-7238. 10.1002/jcp.29622
- RoosterBio. RoosterGEM™: Addressing Key Challenges in Efficient Gene Transfer to Primary Cells. 2021; Available from: https://bit.ly/3NxS5XG.
- Caplan, A. I., Mesenchymal stem cells. J Orthop Res, 1991. 9(5): p. 641-50. 10.1002/jor.1100090504
- Prockop, D. J., Marrow stromal cells as stem cells for nonhematopoietic tissues. Science, 1997. 276(5309): p. 71-4. 10.1126/science.276.5309.71
- Caplan, A. I., Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med, 2017. 6(6): p. 1445-1451. 10.1002/sctm.17-0051
- Viswanathan, S., et al., Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT(R)) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy, 2019. 21(10): p. 1019-1024. 10.1016/j.jcyt.2019.08.002
