“What Are MSCs?” was originally Authored February 15, 2014. Updated by Iain Farrance, Ph.D., and Jon Carson, Ph.D., on February 5, 2020.
Listen to this Blog:
What’s in a Name?
MSC is the common acronym used to describe Mesenchymal Stem Cells or Mesenchymal Stromal Cells. Discussion is ongoing over which long name best describes MSCs — the plastic adherent cells derived from bone marrow, adipose, umbilical cords, or other tissues that can differentiate down multiple tissue lineages (1, 2). While a consensus is developed around a technical long name, the 2005 and 2019 ISCT MSC committee’s position papers recommend that the “MSC” acronym be maintained and appended with the species and tissue of origin (3, 4).
Mesenchymal Stem/Stromal Cells (MSCs), the specialty of RoosterBio are key to many diverse applications in cell therapy and biomanufacturing. RoosterBio is a leading supplier of human MSC working cell banks and hMSC bioprocess systems for research and clinical/GMP use. Our bioprocess media, bioreactors, consumables, protocols, and GMP-certified materials to grow human bone marrow MSCs (hBM-MSCs), human adipose MSCs (hAD-MSC), and human umbilical cord MSCs (hUC-MSCs) are available on our product page.
A Brief History of MSCs
RoosterBio obtains bone marrow from qualified adult donors for its commercial hBM-MSCs. This tissue contains at least two distinct populations of stem cells: Hematopoietic Stem Cells (HSCs) and a rare, plastic-adherent Mesenchymal/Stromal Cell population, MSCs (5, 6, 7, 8). In the bone marrow, MSCs participate in maintaining the blood-forming, or hematopoietic, stem cell niche (7). In vitro, MSCs grow as long, spindle-shaped cells with prominent nuclei and are capable of forming single‐cell colonies on tissue culture plastic. MSCs exhibit potential for self-renewal and the ability to differentiate to various cell lineages including fat, bone, cartilage, and skeletal muscle (5, 9, 10, 11). This multipotency led Arnold Caplan in 1991 to propose the term “Mesenchymal Stem Cells” (5). In addition to bone marrow, MSCs can be isolated from many tissues in the body, including fat, umbilical cord, and dental pulp, to name a few (2).
For universal MSC criteria and to clearly define an MSC, the Mesenchymal and Tissue Stem Cell Committee of the International Society for Cellular Therapy (ISCT) proposed a set of hMSC standards for laboratory‐based scientific investigations and for pre-clinical studies (12, 13). In addition to sterility, stability, potency, and viability, RoosterBio’s RUO hMSCs and cGMP, CliniControl™ (CC) hMSCs are rigorously tested according to these ISCT criteria:
- MSCs must be plastic-adherent when maintained in standard culture conditions using tissue culture flasks.
- ≥95% of the MSC population must express CD105, CD73, and CD90, as measured by flow cytometry. Additionally, MSCs must lack expression (≤2% positive) of CD45, CD34, CD14, or CD11b, CD79a, or CD19 and HLA class II.
- MSCs must be capable of trilineage differentiation in vitro to osteoblasts (bone), adipocytes (fat), and chondrocytes (cartilage).
- MSCs must have immunomodulatory activity, e.g. T-cell suppression via induction of indoleamine 2, 3-dioxygenase (IDO) activity by IFN-γ (±TNF-α).
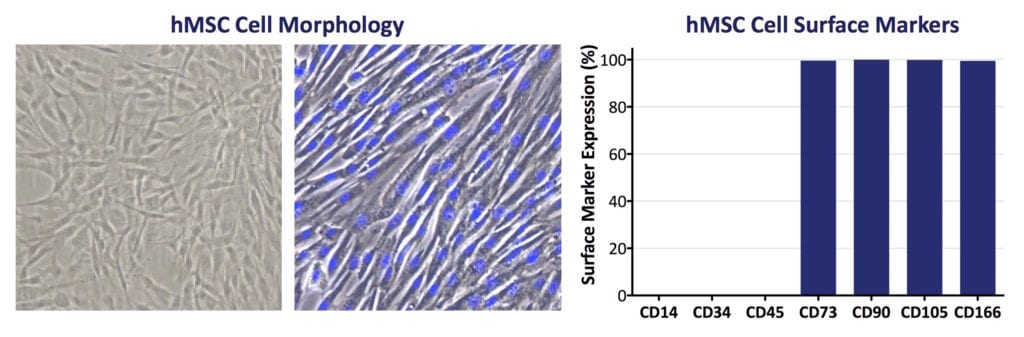
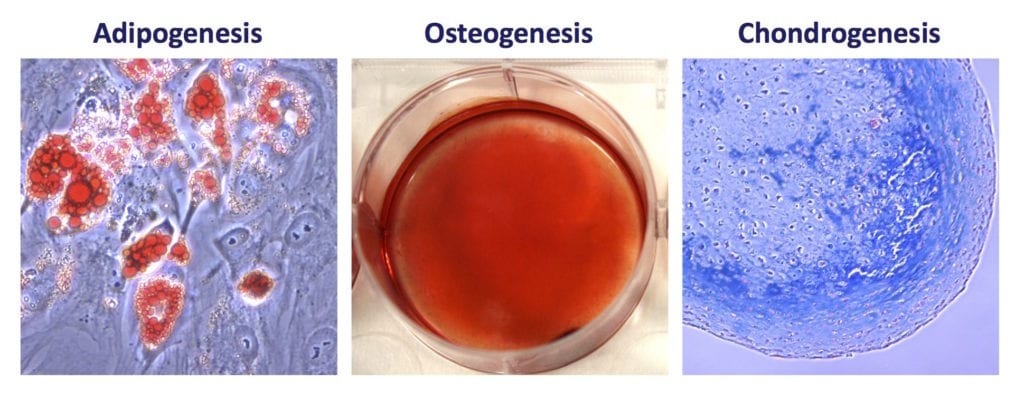
Contemporary MSC Research & Development
While these ISCT criteria established a common framework, these historical criteria have not kept up with how these cells are used in applications (reviewed in 2). For example, RoosterBio recognizes that trilineage differentiation is directly relevant to only a small fraction of all therapeutic applications. Furthermore, flow marker expression has recently been seen as having little relevance to function. For example, CD markers can stay consistent over multiple passages while MSCs lose their differentiation capability or immunomodulatory function (14, 15).
Ongoing research shows that MSCs achieve their therapeutic effects by secreting a plethora of biomolecules and particles that influence many biologic processes (2). Thus, accessing MSC therapeutic relevance is increasingly more focused on secreted biomolecules affecting tissue regeneration or immune system modulation. Additional cell parameters beyond those derived from original standardization literature are, thus, of critical importance to RoosterBio and its customers (12, 14).
A growing MSC research and therapeutic area is MSC-derived extracellular vesicles (MSC-EVs). Extracellular vesicles are a broad category that encompasses exosomes, microvesicles, and apoptotic bodies. MSCs can be prolific secretors of extracellular vesicles, in concert with their physiologic role (16, 17). MSC- EVs can mediate paracrine effects via the transfer of various macromolecules, including intracellular proteins, nucleic acids (e.g., DNA, mRNA & miRNA), metabolites, and membrane-associated factors to target cells. MSC- EVs can be engineered with cell-selective targeting moieties or loaded with artificial therapeutic or diagnostic molecular cargoes. MSC-EVs differ from liposomes due to their natural origin and amenability to robust biomanufacturing. MSC-EVs benefit from their host cells’ well-defined safety profile and are on the rise as a novel clinical therapy for a broad range of applications. Notably, the PubMed query term: “exosome” began to receive more publication hits than “liposome” for the first time in 2019. RoosterBio is developing scalable Extracellular Vesicle tools to empower academic investigators and commercial firms for the EV/Exosome space.
Characterization of cells under Good Manufacturing Practices (GMP) must not only confirm MSC identity (what the cells are) but, must also specify cell potency (what the cells do). The US FDA defines potency tests as measures of appropriate biological activity, with respect to the clinical indication (14). Since many of the therapeutic functions of MSCs derive from their secretome rather than from differentiation capacity, relevant measures of biological function may include ELISA-quantified factors via MSC culture supernatants. In addition, potency tests may measure an induced activity. For example, following migration of RoosterBio MSCs to a site of inflammation or wounding, their response may be to release anti‐inflammatory and/or pro-angiogenic cytokines. Thus, our standardized metric for secretion of VEGF by our MSCs is relevant to their angiogenic potency, and the induction of IDO activity via IFN-γ treatment validates its immunomodulatory function. In vitro potency assays are thus a useful predictive benchmark for in vivo potency to develop cell‐based therapies. They provide a much more clinically-relevant measure of cell function when compared to conventional MSC characterization methods (18). RoosterBio recognizes that cell identity and cell potency are heavily intertwined parameters. Thus, our team characterizes each and every lot of hMSCs for induced IDO activity and a panel of angiogenic cytokines.
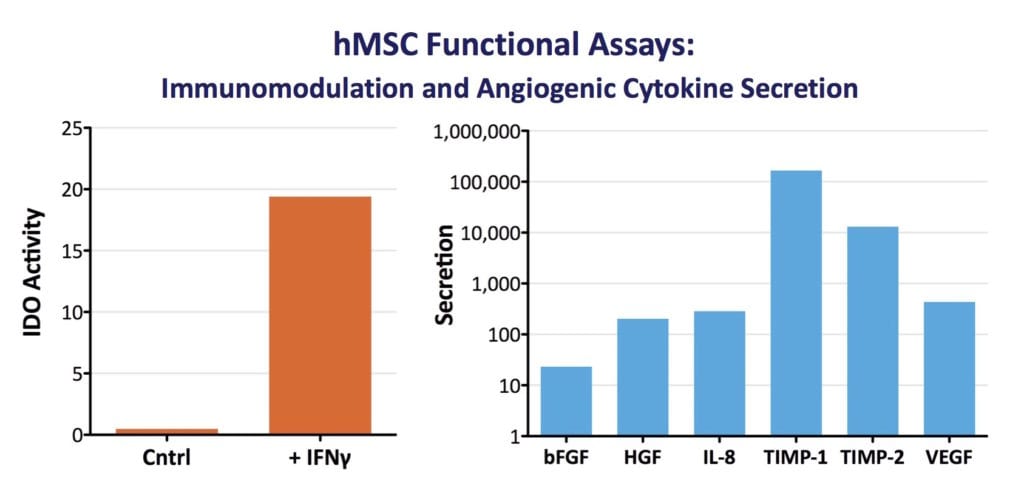
Therapeutic Use of MSCs
MSCs are studied in a wide variety of clinical and therapeutic, including tissue regeneration and wound healing. Structural regeneration and physiologic improvements in damaged and diseased tissues upon therapeutic MSC application were initially attributed to cell engraftment and differentiation within target tissues. Recent results, however, have led to widespread acceptance that most positive outcomes following MSC treatments are due to secreted molecules or MSC-EVs affecting target cell survival and differentiation (2, 16, 19). Clinically speaking, MSCs are not unlike 10000L bioreactors that yield blockbuster drugs such as recombinant insulin, Humira, Keytruda, and Opdivo; however, unlike steel fermenters, MSCs can home to a site of injury and deliver a therapeutic effector in situ within the patient. In addition to MSC’s secretion of trophic factors, the benefits of an established safety profile and lack of permanent engraftment allow for an off-the-shelf administered allogeneic MSC (or MSC-derived) product. Given their widely-noted therapeutic potential, relative ease of isolation, and lack of related ethical concerns, there have been greater than 900 clinical trials initiated using hMSC from various sources (database purchased from celltrials.org). These studies span a host of indications and therapeutic strategies (20, 21) with encouraging results thus far. So, MSCs will continue to be key components of future therapeutic agents, engineered tissues, and medical devices with a “peak” demand many years in the future (22).
Current Bottlenecks in MSC Research & the RoosterBio Solution
Despite their largely untapped therapeutic potential, MSC cells are very rare, comprising only 0.001%‐0.01% of the mononuclear cells in the bone marrow (10). Since a typical adult bone marrow aspirate contains many types of cells and does not yield enough MSCs for a clinical dose, ex vivo expansion of hBM-MSC and other MSC types is necessary before use. However, prolonged culture of MSCs will cause replicative stress, senescence, loss of multilineage potential, and loss of immunosuppressive activity (23, 24). These challenges mean that there is a significant need for efficient methods to expand MSCs ex vivo. Therefore, extensive effort has been expended by RoosterBio and others towards methodology to expand MSCs while maintaining their clinical functionality. Cell plating density, culture surfaces, and the addition of growth factor and cytokine supplements have proven to effectively modulate MSC growth and allow for the uniform expansion of MSCs into clinically relevant lot sizes (See Building Effective Multi-Year Process Development Programs: Estimating hMSC Lot Size Ranges for Clinical Manufacturing Through Commercial Demand and Evolution of Technology Platform Decisions Based on Lot Size).
Product development efforts in cell and tissue engineering, however, face three major challenges today. These are:
- The cost of today’s primary cells is prohibitively high
- Primary cells are not readily available at volumes that translate into the next step of product development efforts. Most cells are offered at less than one million cells per vial at an average cost of over $900 per million cells
- Most product research is performed with cells produced using traditional small-scale processes that are not directly transferrable into a GMP setting – slowing the translation into First-in-Human studies, and eventual commercialization
RoosterBio takes the innovation approach of simultaneous implementation of a number of scalable bioprocess and streamlined improvements. Together, these form a new cost basis for primary cells. Importantly, we perform this without sacrificing product safety, sterility, stability, purity, potency, viability, or identity. RoosterBio’s process engineering innovation of well-characterized research and clinical grade hMSC, from several tissues—paired with highly engineered and standardized media systems—dramatically reduces the total labor hours for MSC culture and the cost per 1M cells. We get customers to the required number of cells 3-4 times faster, at about 40-75% of the overall cost. Thus, as the pace of product development accelerates, we propel the field into a new era of productivity and open up cell- and tissue-based technology development to a much broader market.
References
- Caplan AI (2017) Mesenchymal Stem Cells: Time to Change the Name! Stem cells translational medicine6(6):1445-1451. https://www.ncbi.nlm.nih.gov/pubmed/28452204
- Pittenger MF, et al.(2019) Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med4:22. https://www.ncbi.nlm.nih.gov/pubmed/31815001
- Horwitz EM, et al.(2005) Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy7(5):393-395. https://www.ncbi.nlm.nih.gov/pubmed/16236628
- Viswanathan S, et al.(2019) Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT(R)) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy21(10):1019-1024. https://www.ncbi.nlm.nih.gov/pubmed/31526643
- Caplan AI (1991) Mesenchymal stem cells. Journal of orthopaedic research: official publication of the Orthopaedic Research Society9(5):641-650. https://www.ncbi.nlm.nih.gov/pubmed/1870029
- Friedenstein AJ, et al.(1970) The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet3(4):393-403. https://www.ncbi.nlm.nih.gov/pubmed/5523063
- Friedenstein AJ, et al.(1974) Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation17(4):331-340. https://www.ncbi.nlm.nih.gov/pubmed/4150881
- Friedenstein AJ, et al.(1968) Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation6(2):230-247. https://www.ncbi.nlm.nih.gov/pubmed/5654088
- Caplan AI (1986) Molecular and cellular differentiation of muscle, cartilage, and bone in the developing limb.Prog Clin Biol Res217B:307-318. https://www.ncbi.nlm.nih.gov/pubmed/3092248
- Pittenger MF, et al.(1999) Multilineage potential of adult human mesenchymal stem cells. Science284(5411):143-147. http://www.ncbi.nlm.nih.gov/pubmed/10102814
- Wakitani S, et al.(1995) Myogenic cells derived from rat bone marrow mesenchymal stem cells exposed to 5-azacytidine. Muscle Nerve18(12):1417-1426. https://www.ncbi.nlm.nih.gov/pubmed/7477065
- Dominici M, et al.(2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy8(4):315-317.http://www.ncbi.nlm.nih.gov/pubmed/16923606
- Krampera M, et al.(2013) Immunological characterization of multipotent mesenchymal stromal cells–The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy15(9):1054-1061. http://www.ncbi.nlm.nih.gov/pubmed/23602578
- Carmen J, et al.(2012) Developing assays to address identity, potency, purity and safety: cell characterization in cell therapy process development. Regenerative medicine7(1):85-100.http://www.ncbi.nlm.nih.gov/pubmed/22168500
- Lo Surdo JL, et al.(2013) Automated microscopy as a quantitative method to measure differences in adipogenic differentiation in preparations of human mesenchymal stromal cells. Cytotherapy15(12):1527-1540. http://www.ncbi.nlm.nih.gov/pubmed/23992827
- Elahi FM, et al.(2020) Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem cells38(1):15-21. https://www.ncbi.nlm.nih.gov/pubmed/31381842
- Thery C, et al.(2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles7(1):1535750. https://www.ncbi.nlm.nih.gov/pubmed/30637094
- Galipeau J, et al.(2016) International Society for Cellular Therapy perspective on immune functional assays for mesenchymal stromal cells as potency release criterion for advanced phase clinical trials. Cytotherapy18(2):151-159. https://www.ncbi.nlm.nih.gov/pubmed/26724220
- von Bahr L, et al.(2012) Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem cells30(7):1575-1578. http://www.ncbi.nlm.nih.gov/pubmed/22553154
- Mendicino M, et al.(2014) MSC-based product characterization for clinical trials: an FDA perspective. Cell stem cell14(2):141-145. http://www.ncbi.nlm.nih.gov/pubmed/24506881
- Moll G, et al.(2019) Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines.Trends Mol Med25(2):149-163. https://www.ncbi.nlm.nih.gov/pubmed/30711482
- Olsen TR, et al.(2018) Peak MSC-Are We There Yet?Front Med (Lausanne)5:178. https://www.ncbi.nlm.nih.gov/pubmed/29977893
- Binato R, et al.(2013) Stability of human mesenchymal stem cells during in vitro culture: considerations for cell therapy. Cell Prolif46(1):10-22. https://www.ncbi.nlm.nih.gov/pubmed/23163975
- Li XY, et al.(2012) Long-term culture in vitro impairs the immunosuppressive activity of mesenchymal stem cells on T cells. Mol Med Rep6(5):1183-1189. https://www.ncbi.nlm.nih.gov/pubmed/22923041
