RoosterVial™-hBM-20M-CC
cGMP xeno-free human bone marrow-derived MSCs to support allogenic therapeutic cell manufacturing.
Designed for the regenerative product developer. Fully tested and ready for cGMP manufacturing.
Recommended Products
Overview
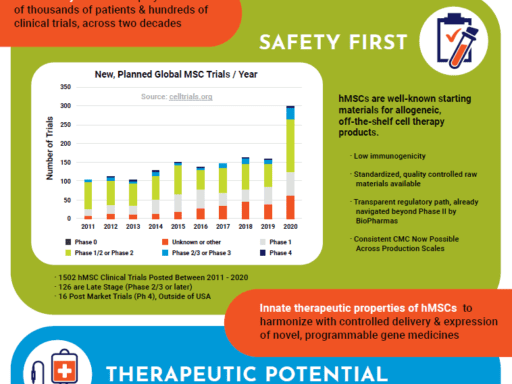
Using a comprehensive and qualified supply chain of our high volume, well-characterized, and standardized WCBs (working cell banks) for large scale cell manufacturing – you’re now able to bypass major steps in cGMP manufacturing. With a rapid 2D process and path to Phase 1 – using 20 x 10-layer vessels, CliniControl™ hMSCs and media get you to the clinic faster, and then scale with your clinical progression to meet your lot size requirements.
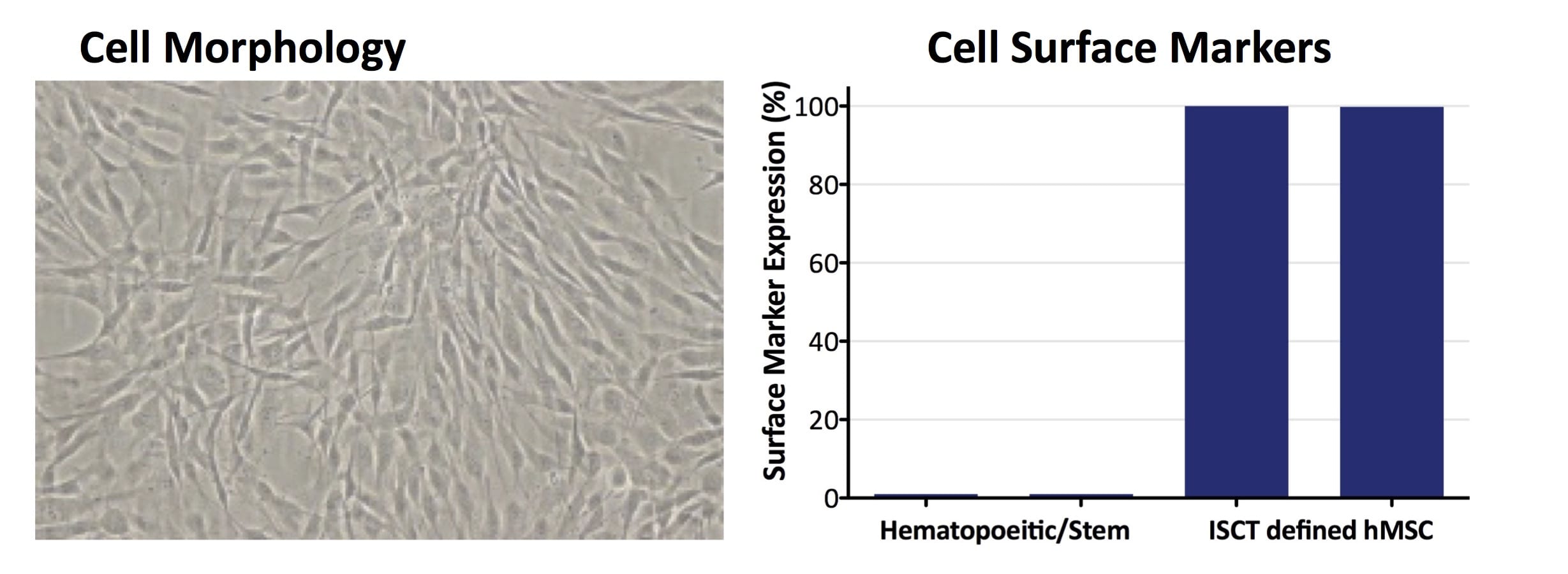
A high quality allogeneic, off-the-shelf WCB for therapeutic cell manufacturing, CliniControl™ (CC) bone marrow-derived hMSCs are manufactured with methods and controls that conform with current Good Manufacturing Practices (cGMP) and are supported for use as a manufacturing starting material by a Master File on file with the FDA. Time spent on investigational new drug (IND) submissions is significantly reduced by facilitating CMC documentation.
To further accelerate your translational research, this clinically-relevant WCB can be tested in development prior to cGMP manufacturing.
Product Features
- 20M cryopreserved XF cells per vial
- Multiple donors manufactured under cGMP
- Sourced under Regional Regulatory guidelines#
- Off-the-shelf WCB – bypasses the need of sourcing tissue, qualifying and releasing your own cell banks
- Consistent manufacturing processes to reproducibly & efficiently scale to billions of cells
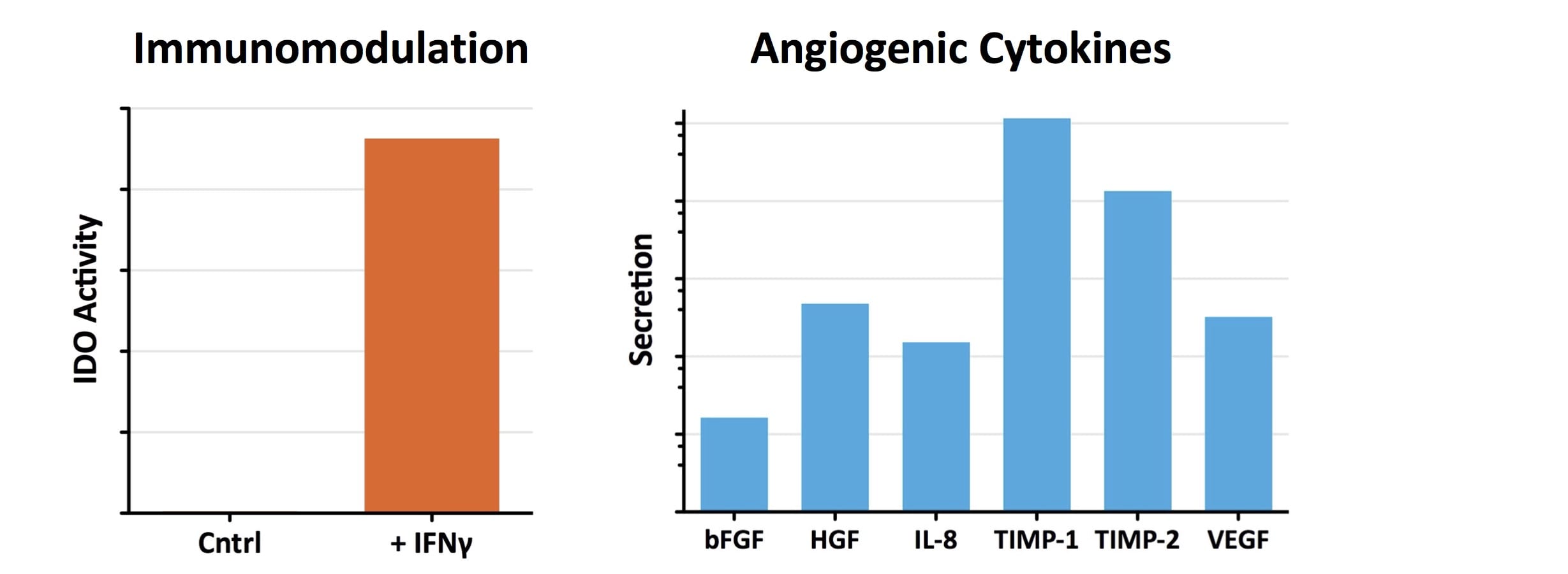
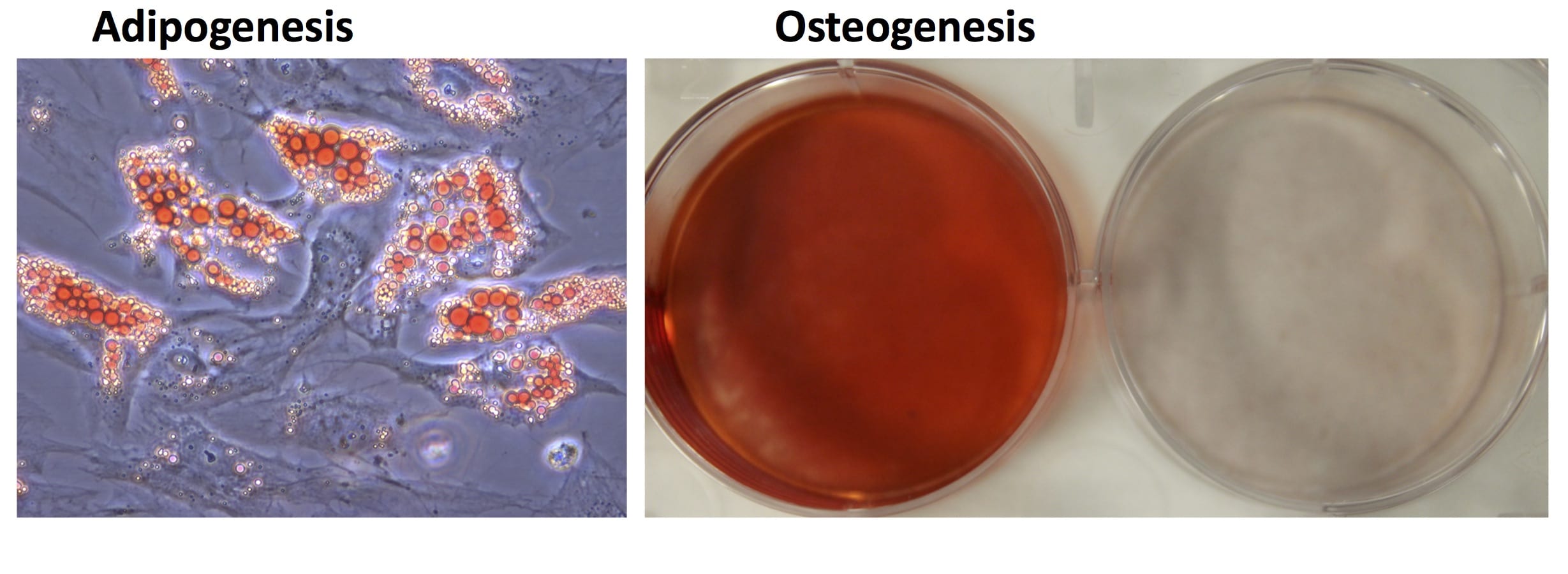
- Industry-leading functional characterization
- Tissue of Origin: Healthy human bone marrow
- Supported by a US FDA Type II Master File for cross reference
- Use with CliniControl™ expansion medium free from media exchange for large reduction in your Cost of Goods
- Seamless 2D (batch) to 3D (fed-batch) culture expansion
- Cryopreserved using a controlled and fully XF
Intended Use: For Further Manufacturing Use Only. Not intended for diagnostic use or for direct human or veterinary therapeutic use.
#CliniControl bone marrow tissue donations comply with US FDA regulations 21 CFR Part 1271 Subparts A-C and applicable sections of Subpart D under Section 1271.150. These products are also manufactured in accordance with European Directive 2004/23/EC and 2006/17/EC. Contact us for additional region specific information.

Protocols and Information
Login to search Certificates of Analysis and Quality Control Briefs.