To Harness Heterogeneity, Extracellular Vesicle (EV) Differences Explored with Deference
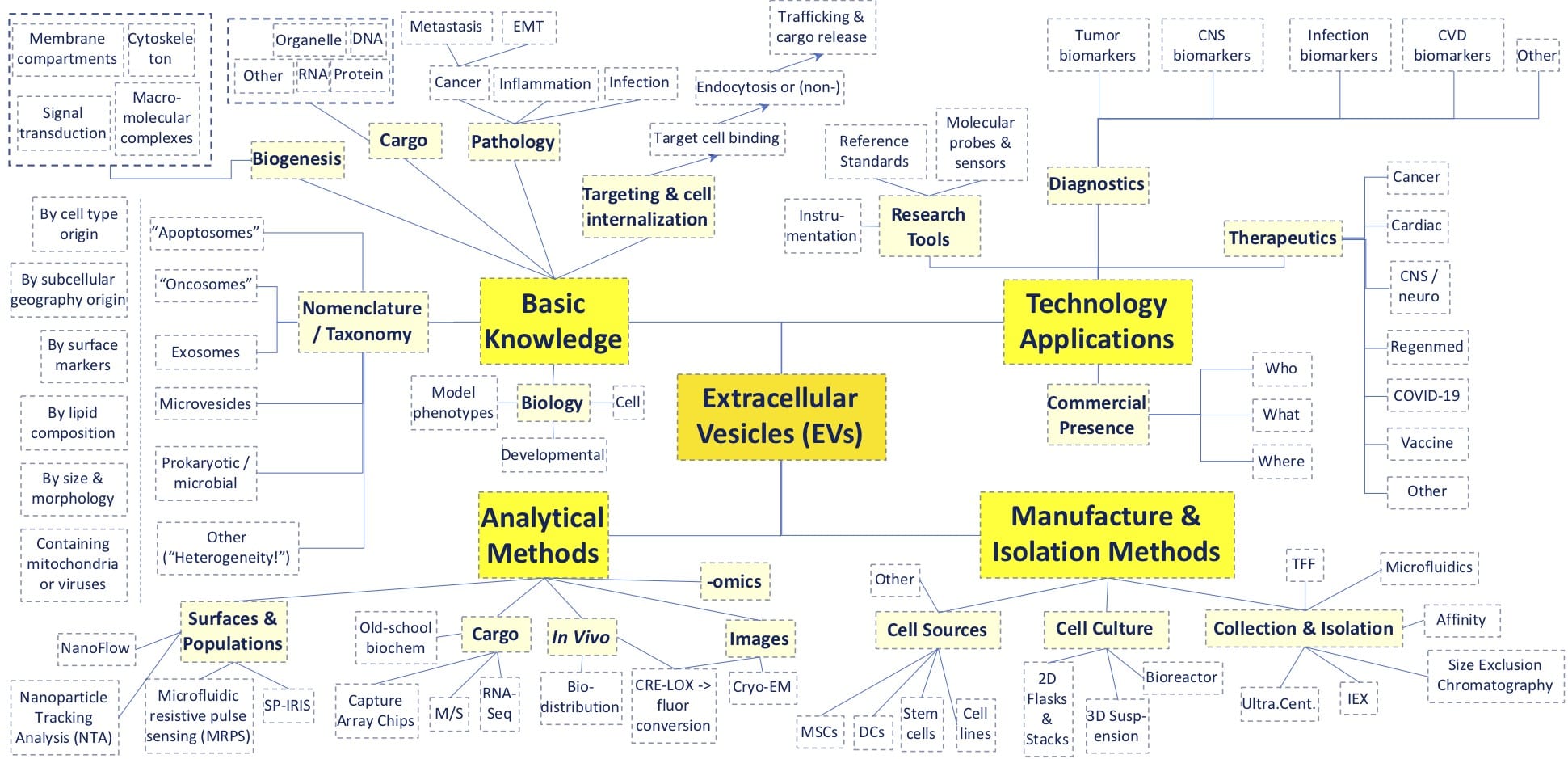 Above, a rudimentary mind-map showing many of the topics on display at 2020’s virtual Annual ISEV Meeting. A major theme was the heterogeneity of different vesicle types and their applicability to human disease and therapeutics.
Above, a rudimentary mind-map showing many of the topics on display at 2020’s virtual Annual ISEV Meeting. A major theme was the heterogeneity of different vesicle types and their applicability to human disease and therapeutics.
And so it begins
According to the Google Dictionary, “heterogeneity” is defined as “the quality or state of being diverse in character or content.” What couldn’t be more diverse than extracellular vesicles (EVs) and their bioproduction sources of origin such as mesenchymal stem/stromal cells (MSCs)? True enough, the recent virtual proceedings (20-July to 22-July) of the annual International Society for Extracellular Vesicles meeting (ISEV2020) launched its kickoff panel discussions with an in-depth look at this very theme. Although there’s surely strength in diversity in a developing field of knowledge, heterogeneity in complex subject material like EVs can present myriad opportunities as well as practical challenges. For developers of EV clinical or commercial products, this digital meeting proved to be an excellent spot to place a finger on the pulse of a fast-paced arena of basic, applied, and translational research. In this blog duet, we therefore attempt to extract some of the key take-home messages of interest to the regenerative medicine opportunity space. In the companion to this blog (Part II), we top off this review with a closer inspection on Manufacturing and Isolation Methods, a focus area of particular interest to RooosterBio.
Professor Clotilde Théry of INSERM at Institute Curie helped set the Conference tone, and laid out these challenges: (i), the many classes of EV exhibit overlapping physical properties such as size, lipid composition, and density; (ii), different isolation methods can yield different subtypes and associated particles from even clonal cell populations; and (iii), different methods of study reveal different kinds of biogenesis, EV cargoes, and interactions with targets. While complex empirical phenomena in science can inspire excellent basic research papers, when precipitated at blizzard rates, might they also place a drag on integration of higher-order knowledge and innovation? To counterbalance this familiar dynamic, the ISEV sets standards to elevate the quality of research practices and material preparations of EVs (see the MISEV2018 Checklist). [1] With added transparency, rigorous controls, and validated analytical and preparative methods, the work shown at ISEV2020 predicts that we’ll have an exciting decade ahead of us, with lots of surprising breakthroughs on the way. As our above “mind-map” illustrates (above diagram), the Conference covered a lot of ground among the more than 1,000 unique institutions and 3,000 author names represented in the >600 posters and oral presentations.
Basic Conference Metrics
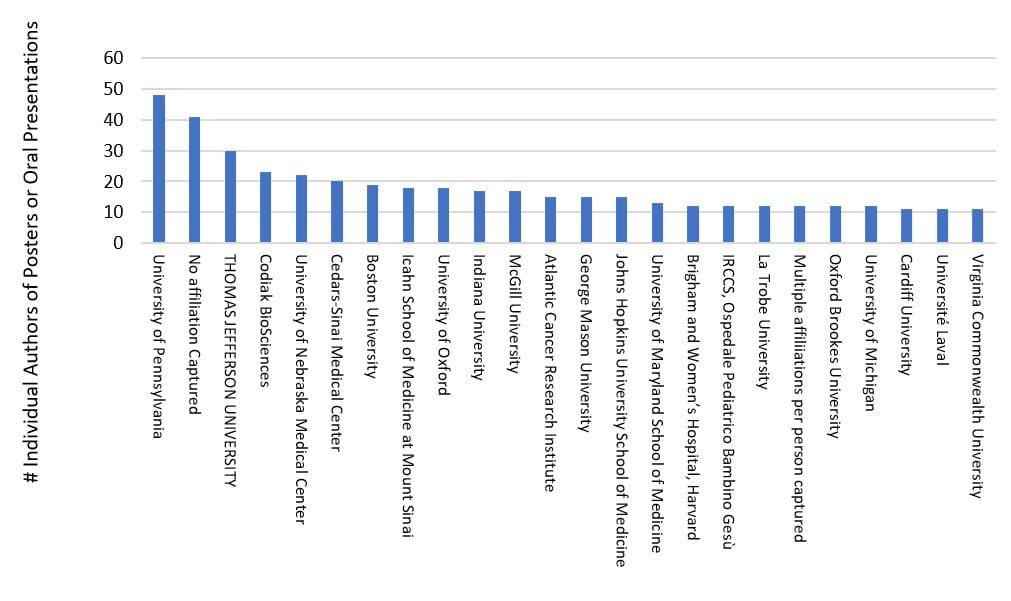
Approximately 30% of the attending institutions provided about 90% of the authored content for this three-day meeting, with strong presence via U. Penn, TJU, Codiak Bio, U. Nebraska, Cedars-Sinai, Boston U., Icahn School of Medicine, U. of Oxford, Indiana U., McGill U., Atlantic Cancer Research Institute, George Mason U., Johns Hopkins U., and many others (see chart above). Symposia, on-demand live talks, and e-posters were categorized around themes of basic EV biology (e.g., nomenclature, biogenesis, cargo, signaling, trafficking, targeting, omics), clinical/translational (e.g., therapeutics, diagnostics, biomarkers, related pathologies & diseases) and technologies/methods (e.g., preparation, cell culture, downstream isolation & purification processes, instrumentation, analytics, and molecular modifications as a controlled delivery platform). The clinical/translational focus was high, providing 47% of the oral presentations and 68% of the posters, followed by technology/methods (34% and 25%, respectively) and basic EV biology (17% and 6% respectively).
By far, most of the attendees hailed from >1000 academic or non-commercial institutions. However, at least 70 company names were present as sponsors, or found on posters and in oral presentations. Of these, most reflected an interest to serve the analytic and preparative needs of EV research. There were also a few up-and-coming therapeutic developers and some established entities such as AstraZeneca, Biogen, Boehringer Ingelheim Pharma, GlaxoSmithKline, and IBM. The latter may well suggest a sign that the EV opportunity space is beginning to ripen. Interestingly, mesenchymal stromal/stem cells (MSCs) appeared in nearly 10% of the posters and oral presentations, indicating a possible trend to incorporate these as prospective starting materials for biomanufacturing of EV products.
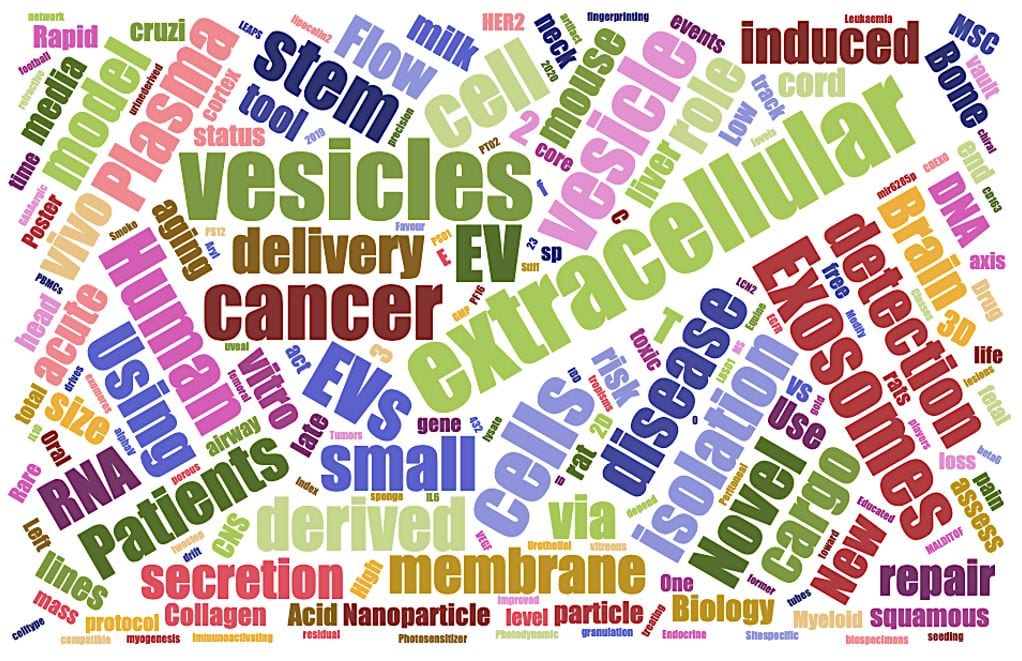
Major related keywords from the poster and oral presentation titles are shown in the above “word cloud” (above from www.jasondavies.com/wordcloud/), with size of word proportional to frequency. In validation of ISEV’s authored content distribution across its category counts, the vocabulary shown here similarly connotes an emphasis on clinical related application—diagnostic or therapeutic (e.g., “human,” “disease,” “vivo,” “MSC,” “brain,” “cancer,” “drug,” “patients,” “pain,” etc.). Many of these words are also of a biological flavor (“model,” “cargo,” “biology,” vault,” “secretion,” “collagen”). As we’ll see in this next blog segment, anything translational or technological ultimately hinges on the biology being well-characterized…
Basic Biology Knowledge
Considering how “heterogeneously” ISEV2020 proceeded, it is impossible to pin down one single breakthrough or over-arching trend in the basic research. Nevertheless, some noteworthy highlights include (i) a growing appreciation for EV cargoes with biological roles once considered atypical—such as mitochondria or viral particles; (ii) an increase in the use of developmental biology toolkits (with higher throughput or faster turnaround time) such as C. elegans, Drosophila, or zebrafish; (iii) compelling new observations on the possible “communication” between different species’ EVs and their cells, e.g., gut-microbiome interactions or plant-host genetic transfers; (iv) insights into the ways EV contents are released into their target cells and where they biodistribute in vivo; and (v) commonalities and differences between EV cargoes (nucleic acid or protein) as they vary between cell/tissue source, disease condition, developmental stage, or biofunction.
Interesting and elegant work that encompassed several of these above themes included “Live tracking of EVs’ life cycle in vivo” (Guillaume van Niel, PhD – Institute of Psychiatry and Neurosciences of Paris), which depicted ongoing study with a live zebrafish model to show the end-to-end choreography of endogenous fluoroprotein-tagged EVs through release, circulation, and uptake. Another was a clever, single-cell reporter system using CRISPR-Cas9, “Uncovering novel genes regulating EV-mediated functional RNA transfer using a CRISPR/Cas9-based reporter system” (presented by Olivier G. de Jong, PhD of UMC Utrecht); this method (“CROSS-FIRE”) loaded released sgRNA into EVs, which enabled “stoplight system” reporter cells to dose-dependently switch Red-to-Green fluoroprotein expression in response to EV uptake and cargo release. Similarly, as Dr. Saumya Das showed us (“Leveraging the exosome-mapping mouse for understanding the functional role of EV signaling”), transgenic mice that express CRE recombinase mRNA in transcriptionally targeted RBCs will generate EVs that map target cells’ distribution in vivo, after cross with a transgenic reporter mouse (with heterologous recombination-activated GFP). These presentations are just three of many examples of where molecular and genetics toolkits, employed in basic biology research, might plausibly scale up into higher throughput (and/or higher content) screening efforts to artificially target therapeutic or “theranostic” exosomes for drug discovery programs. Many of these advances could directly apply to mesenchymal stromal/stem cells, and their incorporation into discovery or preclinical EV product development.
Applied Technology / Translational Applications
As this Conference educated, EV technology can be readily (i) applied into research tools or diagnostics, or (ii) translated into the clinic as a therapeutic. EV research tools include instrumentation systems and their paired consumable reagents, reference standards, and molecular probes. To calibrate analytic instruments and provide computational basis as a “test harness” for comparison or ratioing with unknown EVs samples, defined reference materials of exceptional purity, stability, and uniformity are required. As exampled in Imperial College London’s e-poster (presented by Dr. Richard Kelwick) “Heterogeneity and batch variation of HEK293 extracellular vesicles,” this ambitious goal might be easier said than done, since EV batches from even the same cell line and preparation methods can vary significantly. Yet, speaking as thought leaders in EV reference standards, [2] Professor An Hendrix and her lab (Ghent University) shared their progress via “EV separation methods: variable recovery and specificity requires transparency” and “The use of rEV for the optimization of EV separation and characterization by AF4.” Characterization of reference standards (i.e., rEVs assembled from heterologously expressed GAG-EGFP, which yields traceable and uniform lipoparticles)—plus this group’s establishment of a web-based knowledge depot for transparent method reporting (“EV-Track”) [3]—were messages definitely worth hearing. In parallel, ATCC has also been progressing via its collections of cell lines of unmatched diversity, “including stem cells and cancer cell lines representing the most prevalent cancer types -prostate, colorectal, breast, lung, cervical and glioblastoma,” (see “Evaluation of ATCC’s exosomes from cell culture supernatant as reference standards in research and development)”. With help from these and other advances, we anticipate that the unmet need for highly characterized batches of uniform EVs will be met in the coming decade.
EVs contain (or are tightly bound to) very diverse species of RNA, DNA, protein, metabolites, or even organelles. Since cells in a zone of disease release EVs with distinct cargoes, EV biomaterials likewise yield clues that can be “fingerprinted” analytically and then, further dissected with computational clustering or omics toolkits. Robustly significant indicators out of these panels can, in turn, be adapted for future diagnostic tests. When uniformly collected and isolated, EV samples from whole blood, breast milk, sputum, serum, saliva, urine or other less-invasive biofluids may comprise a bonanza of diagnostic or monitoring biomarkers for “liquid biopsies.” Dozens of Conference presenters thereby sought to address unmet medical needs like cancer (ovarian, metastatic prostate, pancreatic, glioma, etc.), neurodegenerative diseases, traumatic brain injury, kidney disease stage, autoimmune diseases, sepsis, heart failure, and others. For example, Dr. Adries Zijlstra of Vanderbilt “(EV Fingerprinting: Resolving extracellular vesicle heterogeneity using multi-parametric flow cytometry”) spoke about his lab’s work to identify novel biomarker signatures for cancer via CellSTream’s® sensitive CCD-camera enabled flow cytometry to capture across 20-150 features per single EV. Other fascinating work for prospective diagnosis (or monitoring) of coronary disease was shown by Dr. Susmita Sahoo of Icahn School of Medicine (“EV-based diagnostics for cardiac conditions”). Sahoo’s group approached cardiac tissue-specific EV biomarker miRNAs, and found a whole cocktail that were disease-related, a possible breakthrough beyond the present “gold standard” of troponin monitoring for when these miRNAs might be ported together into a high-powered, multiplex readout.
For drug developers, EVs are essentially controlled delivery systems that can be artificially targeted in the body, much like liposomes. To catch up on that topic, “Symposium Session 22: EVs as Delivery Vehicles” is impossible to miss, especially Codiak’s presentation, “Engineering extracellular vesicles with altered cellular tropism for targeted payload delivery in vivo.” Incidentally, ISEV2020 appeared to devote more focus on therapeutics than diagnostics than might be expected out of publication trends (compare PubMed EV queries of therapeutic vs. diagnostic). Given this Conference’s growing focus on EVs for clinical translation, it’s not feasible to distill 100s of presentations into one single message, except one: EV-drugs are advancing rapidly into clinical pipelines. Two exemplars of this message are from MD Anderson Cancer Center’s Dr. Raghu Kalluri (“The Biology and Function of Exosomes in Cancer”) and Capricor/Cedars Sinai’s Dr. Eduardo Marban (“Deconstructing Regenerative Medicine: From Cells to Exosomes and Defined Factors”). Kalluri’s talk provided compelling preclinical data for “iExosomes,” produced under cGMP from mesenchymal stromal/stem cells (MSCs) and cargoed with G12D-Ras-targeted siRNA, vs. pancreatic cancer; these are soon to supply a Phase I clinical trial (link: NCT03608631). Dr. Marban spoke of the rationale for transitioning a cell therapy-based clinical platform (cardiosphere derived cells, CDCs) into a product yielded from EVs/exosomes via immortalized parental cell “factories,” [4] perhaps aimed at Duchenne muscular dystrophy. Marban’s rationale for EVs (after lessons learned from cell therapy trials) is (i) that cells can be a short-lived and fragile material following injection, (ii) that QA/QC and release criteria for cells is more complex from batch to batch, and finally, (iii) that cells behave sub-optimally in closed compartments such as synovium or the eye’s vitreous humor. Specifically, clinical product innovators like Marban ask, can EVs embody the benefits of cell therapy without the potential pitfalls? From a manufacturer’s perspective, however, EVs still remain a highly complex therapeutic class—entirely dependent on a supporting logistics and production process that must still satisfy its own stringent regulatory requirements as a starting material (cells), nourished by controlled and traceable ancillary materials (media). In that sense, we will likely need to be ready for some parallels with the past decade’s lessons from scale up of AAV and lentiviral manufacture for the world’s first marketed gene therapies.
Related to EV use for gene therapy, one fascinating talk builds on the work of Dr. Casey Maguire and colleagues, where AAV-based vectors can incorporate into microvesicles, and thereby be shielded from the common trouble of neutralizing antibodies (NAbs) found in human patients. [5] In turn, Dr. Marta Adamiak (Icahn School of Medicine) showed that these AAVs can package within cardiomyocyte exosomes as “eAAVs,”perform considerably more robustly in animal models of heart function, and are largely unaffected by NAbs. Another cell type of possibly therapeutic relevance to cardiac diseases is the MSC. Incidentally, despite reports of MSCs being challenging to transduce, some variants of AAV can actually enter MSCs efficiently, perhaps raising the prospect that they could someday be employed to help biomanufacture “cloaked” AAVs.
Analytical Methods for EVs
To capture and validate reproducible EV data for basic research, diagnostics, or the clinic, analytical platforms that are both fully standardized and widely adopted need to be established on a steady playing field. By the same token, related instrumentation, biosensor, imaging, and computational tech to characterize EV populations rapidly improves (but regrettably, rarely gets any cheaper). Irrespective of this conundrum, it’s generally accepted that EV preparations for publication need to be described according to their size distribution, morphology, and surface protein expression. Also, the methods to cultivate, isolate, and measure them need to be transparently reported in fastidious detail. ISEV2020 listed hundreds of abstracts that employed methods to analyze EVs at the population and single-particle level, dissect their cargo contents, track their biodistribution in vivo, prepare images of them via electron microscopy, or crunch the dense multi-parameter data into meaningful and reproducible observations.
Although it’s probably premature for a final verdict on competing analytical methods to ascertain population-wide particle metrics, some of the presentations offered us head-to-head comparisons. One notable example of these is “An evaluation of four orthogonal single-particle analysis platforms” presented by Emily Malick of Professor Kenneth Witwer’s lab (Johns Hopkins). Compared were single-particle interferometric reflectance imaging sensor (Exoview®, SP-IRIS) with optional fluorescence, nanoFCM nanoflow (NF), nanoparticle tracking analysis (ZetaView® NTA) with fluorescence, and microfluidic resistive pulse sensing (Spectradyne® nCS1, MRPS). At the end of this talk, there is a good table that compares performance features of these platforms, pertaining to sizing, quantification, and fluorescence, as well as detection range (particle diameter or concentration), total sample volume needed, hands on operation time, and instrument and consumables costs. Each platform varies in ability to quantify and resolve size ranges of mixed populations of particles (although better reference materials are needed). Given different capabilities across different classes of nanomaterials, note Malick’s and Witwer’s key observation that the readout of one instrument type will not readily map to the readout of the other. Finally, while it appears that the NanoFCM gives you the most superior across-the-board performance, its upfront instrument cost is most expensive.
In analyzing single captured EVs, there may be advantages to extract enhanced data features from a set of fewer particles. This tech space, comprising various prototyped microfluidics systems with affinity capture chips, is an exciting one to follow, having originally evolved from efforts to isolate individual circulating tumor cells (CTCs). MGH’s Professor Shannon Stott (“Microfluidics for the Isolation of Extracellular Vesicles”) applies this foundation to EVs, rallying an engineering perspective around this problem with a uniquely interdisciplinary team. Atop this miniaturized platform, it’s possible to optimize capture antibody candidates, driving “bottom-up” efficiency from the nanoscopic level to attain highly sensitive LODs of 100 EVs per 100 uL. Then, one can derive readout data from nucleic acids (hybridizations, amplifications or RNA-seq profiles) or proteins. Such signatures can rapidly monitor the progression or remission of cancer, or discern effects of targeted therapies for personalized drug regimens. As this work continues, it will be interesting to see how initiatives to increase speed and control of individual EVs by tiny-yet-powerful magnetic fields pans out, [6] or perhaps how T2 “relaxometers” could be integrated to yield useful analytic data via mini-NMR. [7] [8] Also importantly, it will be fascinating to observe how these small chips can evolve into manufacturable diagnostic devices to advance human health, or to facilitate in-line bioreactor process quality attributes.
It’s easy to be dazzled by some of ISEV2020’s horizon-crossing techs, but there’s little yet to disrupt fundamental validation methods for EV morphology, which also quietly improves. Is your newly identified particle truly a membrane-bound EV, or just a granular aggregate? Do your EVs actually distribute into the same size range as your instruments report? Do they emerge from the plasma membrane or from multi-vesicular bodies, and where do they traffic when they enter the target cell after host cell release? All these questions are still answered (or confirmed) by the “gold standard,” cryo-electron microscopy (Cryo-EM). “Seeing is believing.” Professor Alain Brisson’s talk (University of Bordeaux; Contributions of Cryo-Electron Microscopy to the EV field) is a good general overview of cryo-EM’s state of the art, which involves characterization of sub-populations with antibody-coupled gold nanoparticles. Electron micrographs continue to be essential for basic research, evaluation of EV isolation procedure methods, biomarker discovery & reagent validation. Of particular interest to EV manufacturers, cryo-EM is needed for quality control of preparation lots for diagnostic or therapeutic applications. In addition to EM, the “old school” Western blot is maintained as a nearly all-essential procedure to validate canonical EV protein composition, to rule out presence of contaminants, and assess the efficiency of fractionations and/or purification steps. As anyone who performs Westerns knows, these assays aren’t known for being particularly high throughput. To ensure that this vital QC step is not a bottleneck for an accelerated, full-scale EV production operation, it’s possible to automate it, as Exosome Diagnostics’ poster shows (“Detection of MISEV recommended EV protein-markers using automated western blotting”).
***
As shown here in this article, ISEV2020 was a fantastic digital venue to be little bit amazed, a little bit enlightened, and a lot motivated to solve some of the basic challenges and questions. A question on everyone’s mind seems to be summed up as “Which EVs are the same and which ones are different?” …A simple question to be sure, but far from answered in any simple way.
In the companion ISEV2020 blog to this writing (Part II), we describe how the Conference addressed how different upstream and downstream EV manufacturing methods can shed additional light on the heterogeneity question, particularly with regard to mesenchymal stromal/stem cells.
References
- Thery, C., et al., Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles, 2018. 7(1): p. 1535750. 10.1080/20013078.2018.1535750
- Geeurickx, E., et al., The generation and use of recombinant extracellular vesicles as biological reference material. Nat Commun, 2019. 10(1): p. 3288. 10.1038/s41467-019-11182-0
- Consortium, Ev-Track, et al., EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Methods, 2017. 14(3): p. 228-232. 10.1038/nmeth.4185
- Ibrahim, A. G. E., et al., Augmenting canonical Wnt signalling in therapeutically inert cells converts them into therapeutically potent exosome factories. Nat Biomed Eng, 2019. 3(9): p. 695-705. 10.1038/s41551-019-0448-6
- Maguire, C. A., et al., Microvesicle-associated AAV vector as a novel gene delivery system. Mol Ther, 2012. 20(5): p. 960-71. 10.1038/mt.2011.303
- Chung, J., et al., Rare cell isolation and profiling on a hybrid magnetic/size-sorting chip. Biomicrofluidics, 2013. 7(5): p. 54107. 10.1063/1.4821923
- Lee, H., et al., Chip-NMR biosensor for detection and molecular analysis of cells. Nat Med, 2008. 14(8): p. 869-74. 10.1038/nm.1711
- Shao, H., et al., Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med, 2012. 18(12): p. 1835-40. 10.1038/nm.2994
