hMSC Analytical Services
Human Mesenchymal Stem/Stromal Cells (hMSCs) are a heterogeneous cell population derived from a variety of tissue and donor sources. As a raw material used by cell, gene, and exosome programs and product developers, understanding the key quality attributes of your hMSC, such as phenotype, function, and growth kinetics are vital to their understanding.
At RoosterBio Inc., we have years of experience in hMSC isolation, manufacturing, and characterization. Leverage this expertise within your hMSC research by contracting our Analytical Services to asses your hMSC cell populations and accelerate your program.
Contact Us For Your Analytical Needs
Our base characterization package includes the following suite of assays:
hMSC Expansion Analysis
The growth kinetics and characteristics of your hMSCs will be evaluated over one passage in xeno-free RoosterNourish-MSC bioprocess media. The analytical output for this assay includes: cell count, morphology, fold expansion, and population doubling level.

MSC morphology of a RoosterVial™-hUC-XF representative sample was visualized throughout expansion to ensure healthy culture along with an example fold increase calculation after 5 days of two hMSC representative samples.
(A-D) RoosterVial™-hUC-XF cells were seeded with brightfield images taken at 4x on days 1, 2, 3, and 4 (respectively).
(E) Calculated fold increase of two hMSC (not RoosterVial™-hUC-XF) representative samples.
Surface Marker Characterization
Using flow cytometry, freshly thawed or passaged Mesenchymal derived cells are characterized as being >90% positive for CD73 (also known as ecto-5’-nucleotidase, produces adenosine), CD90 (Thy1, glycoprotein, role in differentiation), CD105 (Endoglin), and CD166 (membrane glycoprotein ALCAM) as well as being <10% positive for HSC indicators CD34 (hematopoietic marker), CD45 (leukocyte marker), and CD14 (macrophage/monocyte marker).
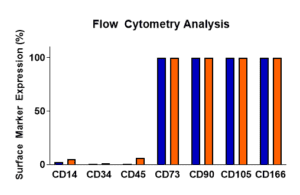
Following a one-passage expansion, representative samples of RoosterVial™-hBM-XF were harvested and evaluated for surface marker expression using flow cytometry.
Immunomodulatory Potential
hMSCs are known for their immunomodulatory effects. Post stimulation with IFN-γ, immunomodulatory activity will be measured by induction of indoleamine 2,3 dioxygenase (IDO) enzymatic activity utilizing a spectrophotometric assay.
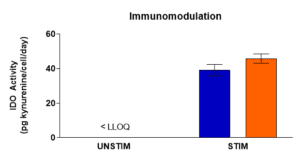
Immunomodulatory activity of representative samples of RoosterVial™-hBM-XF was measured by induction of IDO enzymatic activity following a one-passage expansion.
Angiogenic Cytokine Secretion
Angiogenic activity is important in the treatment of tissue damage and related disease. Using multi-plex ELISA, the angiogenic activity of your cells will be determined by the secretion of cytokine factors bFGF, HGF, IL-8, TIMP-1, TIMP-2, and VEGF.
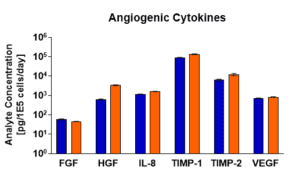
Angiogenic activity of representative samples of RoosterVial™-hBM-XF was measured by secretion of cytokine factors following a one-passage expansion.
Karyotyping
hMSC manufacturing requires large lot sizes which necessitates significant in vitro cell expansion. As a result, chromosomal abnormalities found in cell populations can propagate—causing product and efficacy loss. Our G-band karyotype analysis helps solve for this by identifying these abnormalities early to ensure your starting populations possess chromosomal stability.
We also offer the following add-on characterization service:
Trilineage Differentiation Potential
A hallmark of hMSC identity is their ability to differentiate into adipocytes, osteoblasts, and chondroblasts. These in vitro culture assays confirm the differentiation potential of your hMSCs. Adipogenesis, Osteogenesis, and Chondrogenesis potential of the hMSC is assessed by staining differentiated cells with Oil Red O stain, Alizarin Red stain, and Alcian Blue (respectively).

Following a one-passage expansion, representative hMSCs were seeded and differentiated using: (A) adipogenic differentiation medium, (B) osteogenic differentiation medium, or (C) allowed to form micromasses and then differentiated using chondrogenic differentiation medium.
A) Oil Red O staining of efficiently differentiated adipocytes.
B) Alizarin Red staining of efficiently differentiated osteocytes.
C) Alcian Blue staining of efficiently differentiated chondrocytes.
