RoosterCollect™-EV-CC
cGMP Collection Medium for Exosomes / Extracellular Vesicles (EVs)
Designed for simplified, clean 2D and 3D bioreactor hMSC-EV collection that can be taken into late stage development and cGMP manufacturing.
Recommended Products
Overview
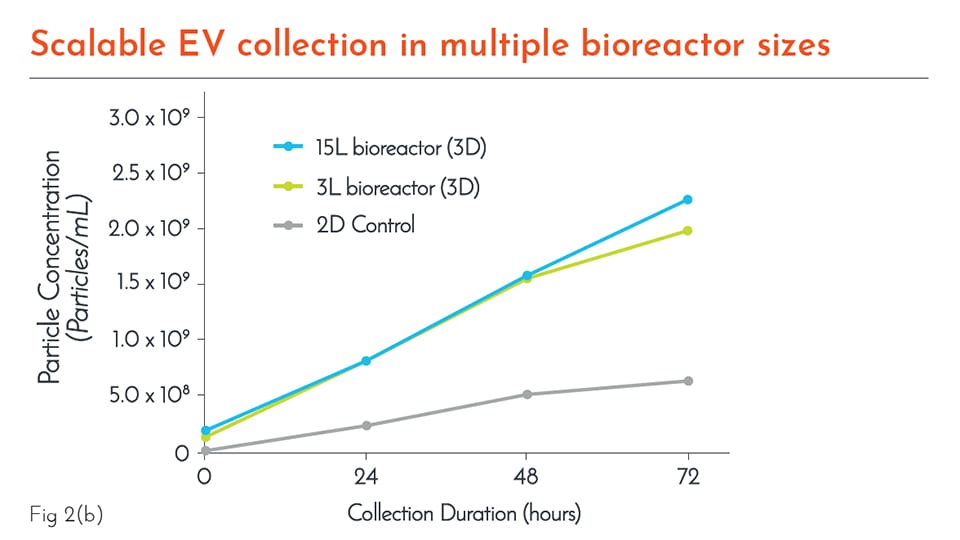
RoosterCollect™-EV-CC is a chemically defined extracellular vesicle (EV) collection media designed to seamlessly integrate into EV, exosome, or cell secretome production platforms. Designed for EV manufacturing across many cell and tissue types in both 2D planar and 3D bioreactor culture conditions, this protein-free and low-particulate media enables “clean collection” resulting in higher yields and easier downstream processing.
RoosterCollect™-EV-CC is easily integrated into existing GMP workflows to support EV collection from preferred cell types, or when combined with RoosterBio’s hMSCs and expansion media, RoosterCollect™-EV-CC turns the RoosterBio cell culture platform into a highly optimized extracellular vesicle production platform.
RoosterCollect-EV-CC is an ancillary material manufactured under good manufacturing practice (GMP) conditions. Regulatory support is available to supplement the chemistry, manufacturing, and control (CMC) section of investigational new drug (IND) applications.
Product Features
- Low-particulate medium, manufactured under cGMP
- EV collection medium in 10L bagged (M02002) or 1L (M02004) bottle format
- Custom formats available
- Protein-free | Chemically-defined
- Per our process recommendations*, this bioprocess system provides scalable and reproducible generation of hMSC-EVs and EVs from other cell types
- Supports flexible system integration to 10-layer vessel and closed system bioreactor processing
*Product needs will be dependent on customer’s process.
Intended Use: For Further Manufacturing Use Only. Not intended for diagnostic use or for direct therapeutic human or veterinary use.
Protocols and Information
Login to search Certificates of Analysis and Quality Control Briefs.
Scientific Resources
Will this product work with my flask or bioreactor system?
The bag was designed to include common connections and weldable tubing to be compatible with a wide range of bioreactor systems and flasks either directly or by accessory parts – some readily available, others that can be designed and ordered through parts distributors. Please, refer to the bag drawing for tubing and connection details to determine the best options for your system.
What material is the bag film comprised of?
The bag film is a polyethylene-based film that has been widely-used in industry for >10yrs. More information including a summary of the validation package can be accessed through customer support, your commercial team representative, and/or during audits.
Will this media work with my cell type?
This EV collection media and the recommended protocol were developed with RoosterBio CliniControl hMSCs and bioprocess media system, but it may work well with other cell types. We recommend testing it out in your system.
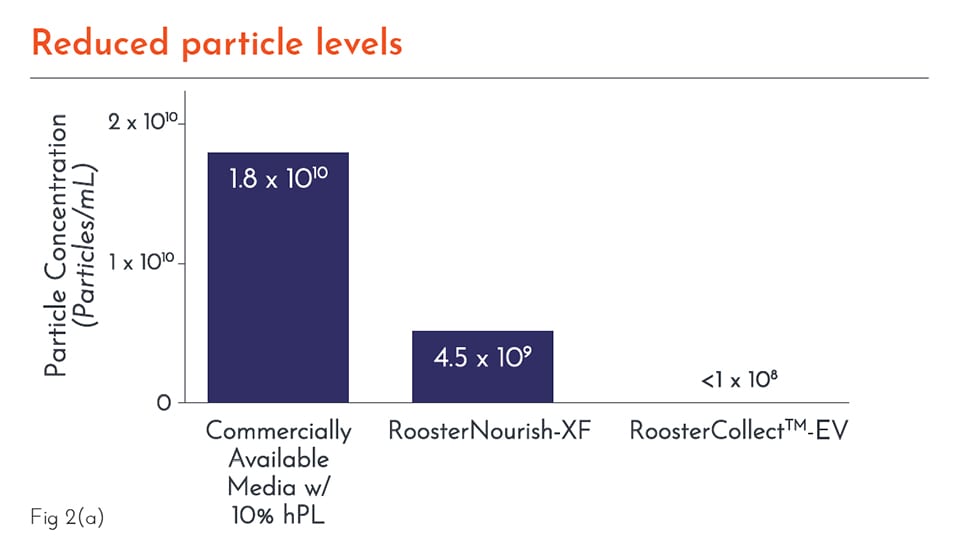
What is meant by a low particulate medium?
Every lot of media is ensured to have a low concentration of particles between 50 and 1,000 nm to ensure that the collected EVs are from the cells and not introduced by the media.