MSC-derived Exosomes
- Collected from Xeno-Free hMSCs & Media
- Produced using RoosterBio’s Optimized 3D Biomanufacturing Process
- >5 Billion particles per vial
- For R&D Use Only
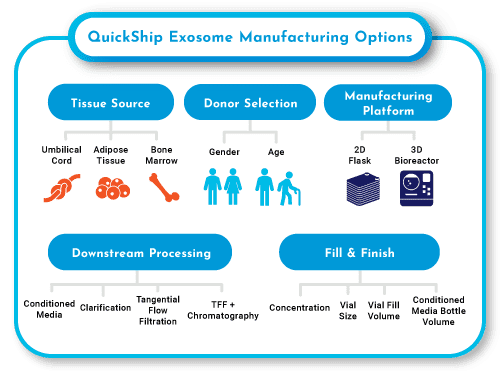
QuickShip™ Exosomes
- Collected from xeno-free hMSCs & media
- Up to 1E15 Extracellular Vesicles per batch
- Multiple tissue sources available
- Customer-defined downstream processing level

Industrialized Exosome Production
Revolutionize the production of exosomes/extracellular vesicles (EVs) with a translation-friendly, scalable bioprocess system designed to drive massive EV yields while minimizing processing time, allowing you to concentrate on your end product. When used alongside RoosterBio’s high-volume xeno-free hMSCs and paired bioprocess media, our EV collection media transfers radically increase EV productivity in both 3D bioreactor and 2D culture applications to meet product development needs and industrialize your extracellular vesicle supply chain.