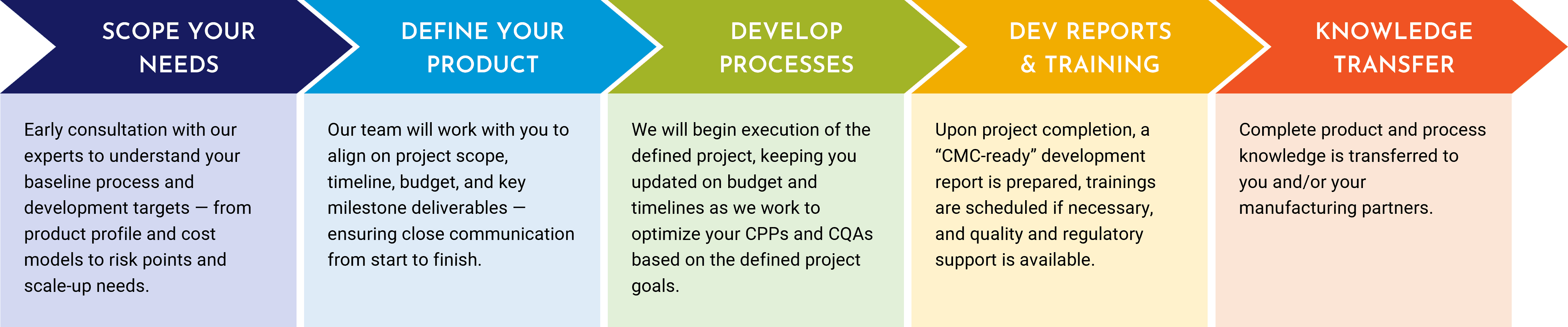
Process Development Services
Upstream Cell & Exosome Process Development Downstream Cell & Exosome Process Development Process Re-Optimization for Established Clinical Programs Genetic Engineering of Cells & Exosomes Stability, Comparability, & Training
Guiding Advanced Therapy Manufacturing
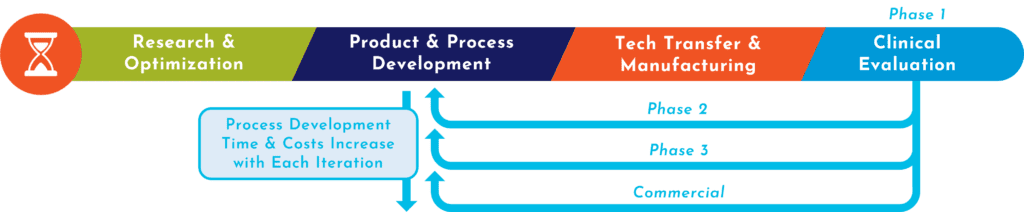
Climbing the unknown without a guide is hard. Similarly, navigating the path from initial concept to first-in-human testing to commercial approval for a cell or exosome-based therapy can be challenging to do alone. RoosterBio has built the products, processes, and expertise to help your advanced therapy program reach new heights — faster and with less risk.
At RoosterBio, we act as a catalyst for advanced therapy Developers, making mountains manageable and accelerating progress to and through the clinic by leveraging over a decade’s worth of process optimization expertise built on a core of technology platforms.
Discover how we can make your mountain easier to climb. Join the growing number of companies benefiting from a customer-centric approach that radically shortens development timelines and the transition to clinical manufacturing. Our mission is to enable your therapy to rapidly reach patients in need.

Develop Successfully Together
Upstream Cell & Exosome Process Development
We offer development labs with industry-leading instrumentation and expertise in upstream manufacturing development and closed system process-scale up. This includes expertise in culture, feed strategy, metabolomics tracking, multi-layer cell culture, 3D microcarrier and suspension vessel culture, and fixed-bed perfusion-based systems. Our facility is equipped with an expanding portfolio of cell culture production systems, such as:
- Adherent 2D Flask & Multi-layer vessel
- Bioreactor Process Development Platforms
- Spinner Flask
- PBS Biotech Pad-Mini Paddle Wheel
- Sartorius Ambr 250 Modular Stirred Tank
- Scalable Bioreactor Development Technologies
- PBS Biotech Vertical Wheel Bioreactors [3L to 80L ]
- Sartorius Biostat STR 50L stirred tank bioreactor
- Eppendorf Bioflo 120 stirred tank bioreactor
- Fixed Bed Bioreactor Development
- Sartorius Biowelder

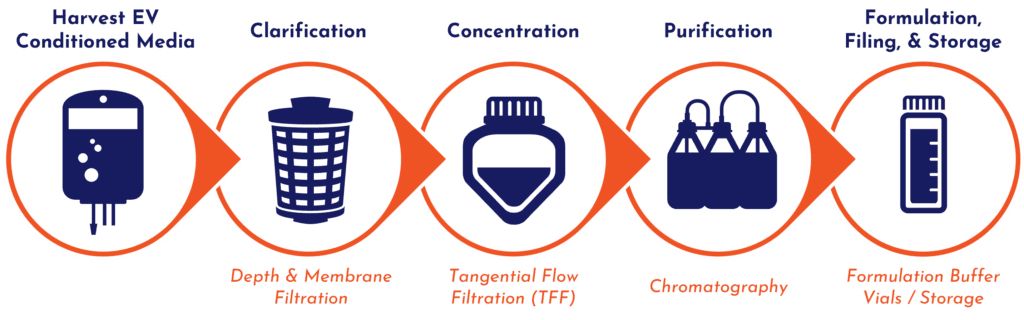
Downstream Cell & Exosome Process Development
Downstream processing can have a significant impact on your final yields, manufacturing lot consistency, and potency. Every cell or exosome therapy is unique; therefore, our process engineers have scalability and customization in mind when leveraging our state-of-the-art equipment to develop a right-fit process from cell harvest and microcarrier separation to exosome clarification, concentration, and purification to final vialing and buffer decisions. When paired with our Analytical Services, we can ensure the process results in a product that meets your criteria.
Our facilities are equipped with:
- Cell harvest and separation: Sartorius KSep
- Exosome/protein concentration: Repligen K22i TFF
- Exosome/protein purification: Cytiva AKTA Start, Cytiva AKTA Avant 150
- Exosome/protein clarification: Repligen TFDF
Process Re-Optimization for Established Clinical Programs
Established clinical programs and licensed therapies engage in process re-optimization to address scalability challenges, costs, raw material changes, or regulatory considerations. Changes at this stage require a significant ROI to offset costs and risk. Let our experts outline and develop a program designed to maximize the efficiency of change while at the same time considering regulatory implications and the CQAs of your therapy.
Process re-optimization and paired bridging studies:
- Improve the regulatory profile of raw materials used in manufacturing.
- Address changing regulatory requirements or enter new markets.
- Increase production scales to meet growing dosage demands.
- Improve manufacturing to lower cost per billion/trillion cells or exosomes produced.
Genetic Engineering of Cells & Exosomes
Genetic engineering and modification (GE)-based approaches are being used for a growing number of targets and rare indications, previously untreatable by current technologies, opening a path for new “next-generation” cell and exosome-based therapies. The transition from proof-of-concept is bottlenecked by gene-transfer efficiency challenges required to meet scales and costs needed for autologous or allogeneic therapy success.
Leverage our growing GE solution set to develop processes to manufacture your specific gene-modified cell or exosome. We have process knowledge with genetic engineering media solutions in an expanding number of cell types and application areas, such as:
- Lenti-viral transduction
- mRNA transient transfection
- siRNA transfection
- AAV transduction
- Exosome engineering and modification
- Primary cell engineering
- Genetic engineering/expansion optimization
Stability, Comparability, & Training
From training to comparability and stability studies, there are many ways RoosterBio is utilizing our expertise to support advanced therapy developers. Program needs range from full process and product development to targeted study design and execution. Leverage our experts to outline your targeted critical data generation needs, such as:
- Bridging and comparability studies for:
- 2D to 3D transition
- Manufacturing platform changes
- Cell bank and donor comparison
- Media comparison
- Cost of goods analysis
- Evaluation and consultation for regulatory feedback study design
- Assay development for residuals
- Stability program design and management
Completion of the final study or report does not end our support. Explore opportunities for training and education on the platform knowledge needed to successfully implement and operate upstream, downstream, and analytical equipment. Training is available on our suite of bioreactor and DSP platforms, as well as customizable on-site or remote training to provide process-specific instruction or to facilitate Tech Transfer to a CMO partner.
