Empower Your Therapeutic Development

An industrialized supply chain of high-quality hMSCs paired with productive bioprocess media and key process parameters can fuel hundreds of next-generation cell-based therapeutics on the horizon. We utilize our core products and expertise to help develop cell-based therapies with commercially viable biomanufacturing processes, right-sized to your needs.
- Scalable hMSC and Exosome Product Systems
- Designed for 2D and 3D Processes
- Facilitating Research and cGMP Manufacturing
More Cells in Less Time
Highly Productive Products
Billions is the new millions for consistent, high-quality cells
Lowest Cost per Million Cells
Commercially viable cost structure scalable from 2D to 3D
Standardized Bioprocess Systems
Off-the-shelf products with faster, easier protocols and reduced risk
Reduced Product Development Timelines
A clear path from research to cGMP manufacturing in a fraction of the time
Our hMSC Systems
No Matter Your Path There's a Built-in Manufacturing Process
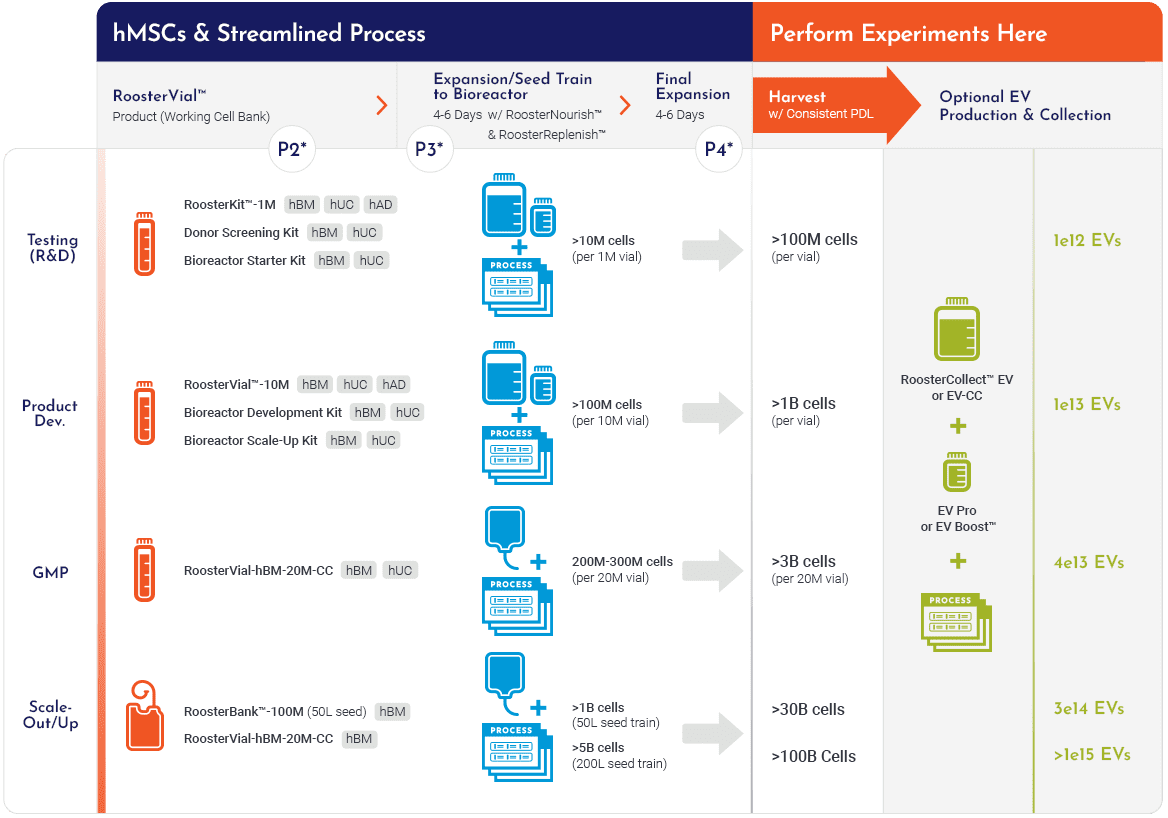
*Expected hMSC and EV yields are approximates for an average hBM-MSC donor and can vary for different donors and tissue sources. Details on expected yields for other tissue sources are available upon request.
Technology Supported By Experience
Alongside our innovative core technology is years of industry experience to support your hMSC-based research and manufacturing. Work with us to radically shorten your development timelines and accelerate your path to commercialization.
New to hMSC Product Development?
We've written a few articles to help out.