Part I of a Blog Series on CQAs & CPPs
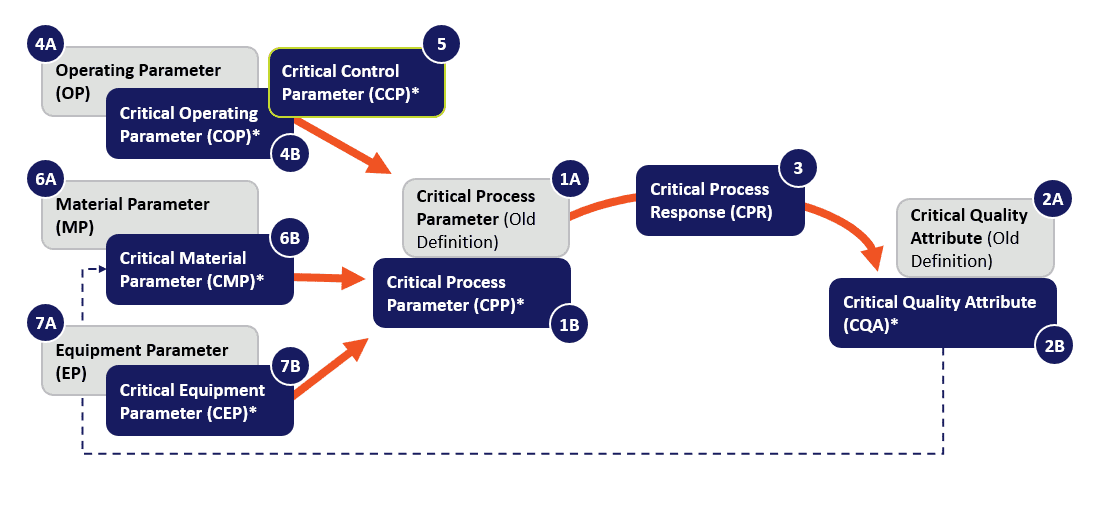 Above, A diagram of current and proposed definitions to govern bioprocess development planning, conceived as a cycle with amplifying feedbacks, not a linear process.
Above, A diagram of current and proposed definitions to govern bioprocess development planning, conceived as a cycle with amplifying feedbacks, not a linear process.
“As we know, there are known knowns; there are things we know we know. We also know there are known unknowns; that is to say we know there are some things we do not know.”
– Donald Rumsfeld, 2002
Who knew that the former Secretary of Defense was ghost-writing a blog about controlling the “known unknowns” to minimize product variability via dynamic bioprocess controls? …Well, perhaps not, but that oft-quoted comment does serve to inform the truth that even the unseen can be positively influenced—when the correct monitoring and controls are in place.
Cell and gene therapies (CGTs) are among the most complex medicines in terms of both their desired mechanism and especially their ontology. They are thus more challenging to produce, test, and bring to market than small molecules or even protein biologics. [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] While the safety record of most of these has been very good, we’ve observed confounding and costly failures in mid-to-late Phase trials, due to evaporation of initial positive signs of efficacy. Could it be that variations in process (e.g., during manufacturing scale-up or scale-out) account for some of these higher p-values and elusive trial endpoints? Dr. Mark F. Witcher has written expertly on this topic in his March 2020 editorial entitled, Why Controlling CQAs Isn’t Good Enough For Gene & Cell Therapies. [12] [13] [14] [15]
|
Critical Process Parameter (CPP) “A process parameter whose variability has an impact on a critical quality attribute and therefore should be monitored or controlled to ensure the process produces the desired product quality.” |
Critical Quality Attribute (CQA) “A physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality.” |
Above, Existing concepts from Agency guidance for drug quality.
In his writing, Witcher states that the CGT development space needs to adopt more rigorous working definitions beneath the two over-arching regulatory terms [16] provided for guidance in the design and control of drug quality: the Critical Process Parameter (CPP) and the Critical Quality Attribute (CQA). Simplistically stated, the CPP is like the lever or “cause” – and the CQA is the readout or “effect.” Yet these current definitions are ambiguous, and they fail to properly educate newer biotech innovators—resulting in confusion, lack of transparency, and clinical product yields of uneven safety and efficacy across “similar” manufacturing processes. Witcher convincingly argues that while it’s well and good to track the known CQAs, it’s also necessary to deconvolute their meaning with more sophistication than in the past. Perhaps this Industry needs to do a better job to diminish the impact of “unknown” outputs i.e., through stringent process control over “knowns” via the process. Through this control, certain downstream attributes that Witcher coins as “u-CQAs” would be less inclined to wreak havoc. He asserts, “The key concepts in the new definition are that CQAs can be both measurable and unmeasurable and that u-CQAs can only be controlled and therefore must be controlled by the manufacturing process.”
Yet how is this doable? Especially for complex CGTs, it’s admittedly impossible to track each and every last CQA to the ends of the earth! The answer is both simple and complex: the CQA ought to better blend with the process itself to infuse it with more robustness, i.e., resistant to perturbations. A keyword is “control.” Here is Witcher’s proposed revised definition for the CQA, with new words added bold in the text:
Critical Quality Attribute (CQA)*- A measurable or unmeasurable physical, chemical, biological, or microbiological property or characteristic that should be controlled by the manufacturing process to be within an appropriate limit, range, or distribution to ensure the desired product quality.
Hence, when the complex, end-to-end CGT drug manufacture workflow is viewed as a whole, one step’s CQA can directly feed into the next step’s critical process parameter, and into the next CQA, and so on. It is not a linear flow, but rather an iterative cycle. Thus, considering CPPs, it’s necessary to carefully account for all possible “inputs” and “outputs” into the bioproduction engine. Certain dynamic parameters of the bioprocess, itself, can be (and often are) monitored and responded to as “output” process parameters. Their near-real-time or in-line readout can, in turn, be reported as CQAs in the revised schema, defined by Witcher as the “CPR”:
Critical Process Response (CPR)* – A output process parameter whose variability has or may have an impact on a measurable or unmeasurable critical quality attribute and therefore should be monitored or controlled to ensure the process produces the desired product quality.
Now let’s consider inputs. Although input parameters for critical process parameters have been described before as “Operating Parameters (OPs),” “Material Parameters (MPs),” and “Equipment Parameters (EPs),” Witcher asks the CGT manufacturer to take another careful look at their process flow diagrams, and consider updated definitions for these inputs in a new light, by emphasizing the added term “Critical” suffixed into each of these, i.e., COPs, CMPs, and CEPs. By “critical,” it is meant that “uncontrolled variability may have a negative impact” on quality, and should therefore be “appropriately monitored or controlled to ensure” that the process yields a desirable standard of product. Since biological manufacturing uses multiple parallel sub-processes that combine into dependent downstream ones, it’s not difficult to observe how the CQA of an earlier process can be a defining CMP of the next one. We are used to thinking of CQAs as being downstream. Witcher describes them as integrated into a larger whole.
The notion that Biology can be “fully” controlled as a closed system is misleading. In a commercial bioreactor, there are billions of unique nucleoplasms, each one the “control center” in billions of individual, heterogeneous “factory” cells that act together in a non-linear ecosystem. To police them all during the end-to-end process surely requires the serenity to accept the things that cannot be changed, the courage to change the things that can, and the wisdom to know the difference. Where and when can we start to manage risk effectively? We do it with good (or better) definitions written into the process plan. Dr. Witcher insightfully concludes:
“The restructuring of the definitions of CQA and CPP is part of evolving ICH Q8 into a well-structured design space to better define and understand unit operations for using process‑based risk analysis and life cycle process development and validation methods. While regulatory agencies may not change or modify their current definitions of CQA and CPP, understanding their underlying concepts as expressed in the alternative definitions is critical to properly developing complex biopharmaceutical processes like those required to successfully commercialize gene and cell therapy products.”
In Part II of this blog series, Critical Quality Attributes: Know Their Importance & Limitations in Product & Process Development, we’ll be writing in greater detail about process input and output controls, and how we have begun to adroitly grasp them via our bioprocess engineers who work to incorporate these concepts into our own scale-up bioreactor processes for hMSCs.
At RoosterBio, we offer a wide range of development services that will help you understand the “known unknowns” that affect your critical process parameters & critical quality attributes.
Below, A glossary of terms as defined by M. Witcher. [15]
Term |
Definition |
| 1A. Critical Process Parameter (CPP) | A process parameter whose variability has an impact on a critical quality attribute and therefore should be monitored or controlled to ensure the process produces the desired product quality. |
| 1B. Critical Process Parameter* New Definition | An input or output process parameter whose variability has an impact on a critical quality attribute and therefore should be monitored or controlled to ensure the process produces the desired product quality. |
| 2A. Critical Quality Attribute (CQA) | A physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality. |
| 2B. Critical Quality Attribute* New Definition | A measurable or unmeasurable physical, chemical, biological, or microbiological property or characteristic that should be controlled by the manufacturing process to be within an appropriate limit, range, or distribution to ensure the desired product quality. |
| 3. Critical Process Response (CPR) | An output process parameter whose variability has or may have an impact on a measurable or unmeasurable critical quality attribute and therefore should be monitored or controlled to ensure the process produces the desired product quality. A measurable CQA may be used as a CPR. |
| 4A. Operating Parameter (OP) | An input process variable that is manipulated manually or by automated control systems during process development and manufacturing operations, to control the process performance and thus product quality |
| 4B. Critical Operating Parameter (COP) | An input process parameter defined and validated during process development used to define and control the process’ behavior, whose uncontrolled variability may have a negative impact on a critical quality attribute and/or critical process response and therefore should be monitored and controlled to ensure the process produces the desired product quality. E.g., media composition, buffer concentrations, mixing rates, gas addition rates, etc. |
| 5. Critical Control Parameter (CCP)* | A critical operating parameter (COP) that is manipulated based on a measured critical process response (CPR) or other input parameter to control the process’ performance such that it remains within appropriate limits, ranges, or distributions to ensure the desired product quality. |
| 6A. Material Parameter (MP) | the attributes of the raw material or in-process materials that feed the process. Examples of MPs include the physical, chemical, biological, or microbiological property or characteristic of the incoming materials such as impurities and contaminants, along with chemical composition. For a manufacturing process, some of the MPs could be the critical quality attributes from the prior process unit operation. |
| 6B. Critical Material Parameter (CMP)* | A measurable or unmeasurable physical, chemical, biological, or microbiological property or characteristic of an input material whose value and variability has an impact on a critical quality attribute and/or critical process response and therefore should be appropriately monitored or controlled to ensure the process produces the desired product quality. E.g., chemical composition, impurities, and contaminant levels |
| 7A. Equipment Parameter (EP) | the parameters of the equipment, including size, capacity, materials of construction, etc. EPs are usually defined in the equipment’s user requirement specification (URS). Particular attention should be paid to EPs that might change during operation, such as heat transfer capabilities that might change because of fouling. Some EPs should be monitored and controlled by a variety of maintenance control strategies. |
| 7B. Critical Equipment Parameter (CEP)* | A process equipment design parameter whose value or variability has an impact on a critical quality attribute and/or critical process response and therefore should be appropriately defined, monitored, or controlled to ensure the process produces the desired product quality. E.g., volumes, materials of construction, agitator type, heat transfer area, etc. |
References
- Dimmeler, S and Leri, A, Aging and disease as modifiers of efficacy of cell therapy. Circ Res, 2008. 102(11): p. 1319-30. 10.1161/CIRCRESAHA.108.175943
- Behfar, A, et al., Cell therapy for cardiac repair–lessons from clinical trials. Nat Rev Cardiol, 2014. 11(4): p. 232-46. 10.1038/nrcardio.2014.9
- Celladon’s Mydicar Fails Phase IIb Trial. GenEngNews 2015; Available from: https://www.genengnews.com/topics/genome-editing/celladons-mydicar-fails-phase-iib-trial/.
- van der Loo, JC and Wright, JF, Progress and challenges in viral vector manufacturing. Hum Mol Genet, 2016. 25(R1): p. R42-52. 10.1093/hmg/ddv451
- Baker, M, Reproducibility: Respect your cells! Nature, 2016. 537(7620): p. 433-5. 10.1038/537433a
- Chinnadurai, R, et al., Potency Analysis of Mesenchymal Stromal Cells Using a Combinatorial Assay Matrix Approach. Cell Rep, 2018. 22(9): p. 2504-2517. 10.1016/j.celrep.2018.02.013
- Galipeau, J and Sensebe, L, Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell, 2018. 22(6): p. 824-833. 10.1016/j.stem.2018.05.004
- Adams, B. Sarepta DMD gene therapy hold lifted in ‘record time’. FierceBiotech 2018; Available from: https://www.fiercebiotech.com/biotech/sarepta-dmd-gene-therapy-hold-lifted-record-time.
- Johnston, PV, et al., Not All Stem Cells Are Created Equal. Circ Res, 2018. 123(8): p. 944-946. 10.1161/CIRCRESAHA.118.313425
- Robb, KP, et al., Mesenchymal stromal cell therapy: progress in manufacturing and assessments of potency. Cytotherapy, 2019. 21(3): p. 289-306. 10.1016/j.jcyt.2018.10.014
- Godoy, JAP, et al., Clinical Translation of Mesenchymal Stromal Cell Therapy for Graft Versus Host Disease. Front Cell Dev Biol, 2019. 7: p. 255. 10.3389/fcell.2019.00255
- Witcher, MF, Integrating Development Tools into the Process Validation Lifecycle to Achieve Six Sigma Pharmaceutical Quality. BioProcessing Journal, 2018. 17.
- Witcher, MF. The Facility Challenges of Developing Continuous Process based Biopharmaceutical Products. ISPE.org 2019; Available from: https://ispe.org/pharmaceutical-engineering/ispeak/facility-challenges-developing-continuous-process-based-biopharma-products.
- Witcher, M. Phase III Clinical Trials – Ever Wonder Why Some Products Unexpectedly Fail? Pharmaceutical Engineering 2019; Available from: https://ispe.org/pharmaceutical-engineering/ispeak/phase-iii-clinical-trials-ever-wonder-why-some-products-unexpectedly-fail.
- Witcher, M. Why Controlling CQAs Isn’t Good Enough For Gene & Cell Therapies. Cell & Gene 2020; Available from: https://bit.ly/2xBvCHj.
- FDA. Guidance for Industry – Q8(R2) Pharmaceutical Development FDA.gov 2009; Available from: https://www.fda.gov/media/71535/download.

