RoosterBio Quality Excellence: We nurture a hard-working innovative culture where quality begins at inception.
RoosterBio’s mission is to Fuel the Rapid Commercialization of Scalable Regenerative Cures. We contribute to this purpose by providing off-the-shelf starting and ancillary materials for further manufacturing of cell or gene therapy products. RoosterBio ensures that quality is built into our cell and media products to enable you to obtain your final cell or gene therapy product goals more rapidly. Quality Assurance and Quality Control measures are taken at every stage of our products’ life cycle beginning with product inception. In this post we provide a high-level overview of the mesenchymal stromal cell (MSC) CliniControl™working cell bank product manufacturing, highlighting the key quality controls and assurances.
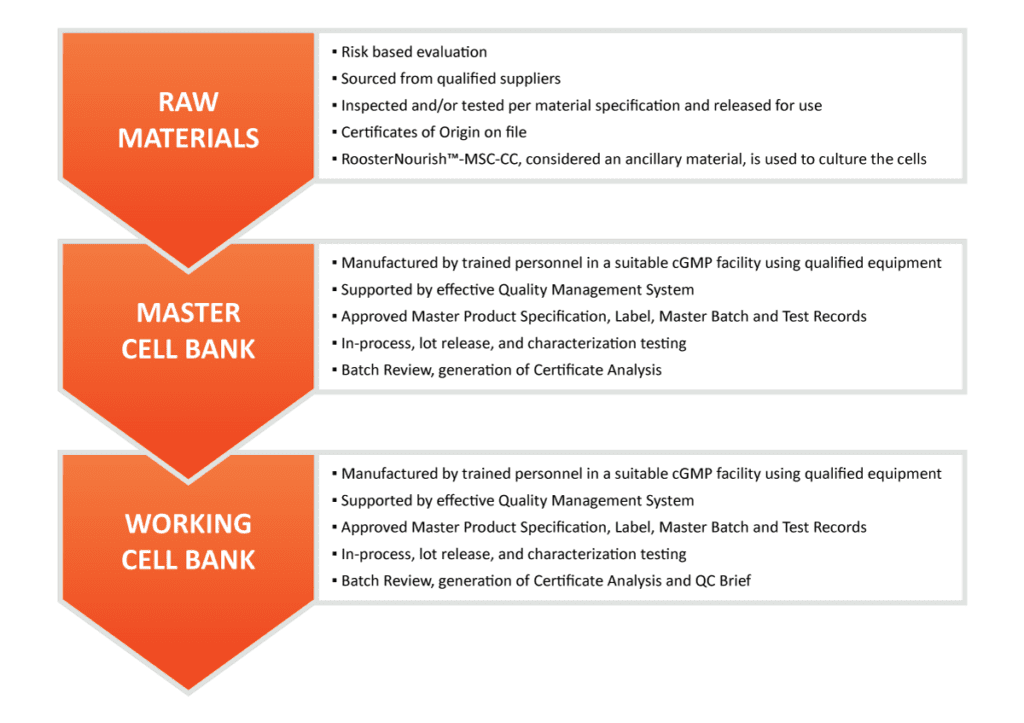
Quality Starts With Our Suppliers & Raw Materials
Quality considerations come into play early with the selection of raw materials in product and process development. The supplier itself is just as critical, if not more so, than the actual raw material. Here at RoosterBio, we adhere to our effective vendor qualification program including assigning a risk category to raw materials and components, auditing suppliers to evaluate their Quality Management System and facilities against the principles of applicable cGMP standards, and continually monitoring vendors to maintain a state of acceptable qualification. A robust vendor qualification management program is critical to mitigating risks down the road.
Xeno-free Products: Critical Human Derived Raw Materials
The starting human tissue (i.e., bone marrow aspirate) from which mesenchymal stromal cells (MSCs) are isolated is a critical raw material. Here in the US, our starting tissue must meet federal regulations set out in 21 CFR 1271, Human Cells, Tissues, and Cellular and Tissue-Based Products. Similarly, the donors of human blood-derived raw material in our media are tested in compliance with federal regulations set out in 21 CFR 610.40, Testing Requirements for Relevant Transfusion-Transmitted Infections. Global regulations for human-sourced biological raw materials vary by region and country. Understanding and keeping up with evolving regulatory requirements is key to our customer’s successful approval of their final product.
RoosterBio’s MSC banks are aseptically manufactured in clean rooms in compliance with current good manufacturing practices. This includes trained personnel, a controlled environment, qualified and calibrated equipment, adequate production and process controls, and approved documentation.
From Tissue to Cells: Creating a Master Cell Bank
Our master cell banks (MCBs) are generated from fresh tissue sourced from eligible donors. The MSCs are isolated, expanded, harvested, vialed, labeled, and cryopreserved. MCBs then undergo extensive confirmatory safety testing. The entire batch file is reviewed by the Quality Department per standard operating procedures and released with a lot-specific Certificate of Analysis (CoA).
From Master Cell Bank to Working Cell Bank
Master cell bank vials are thawed and expanded to create the working cell bank, (i.e., RoosterVial-hBM-20M-CC). Multiple WCB lots can be produced from the same MCB providing a large supply from a desired donor. Each lot is evaluated for quality attributes that include safety, identity, quantity, viability, and markers of biological activity as reported on lot-specific CoAs. In addition, RoosterBio also provides to customers a QC Brief which summarizes additional characterization testing conducted on each lot including trilineage differentiation potential, a measure of Immunomodulatory Expression, and population doubling level. Our goal is to continue to build up our high-quality WCB portfolio of donors and provide our customers the information they need to make the best choice for their indication.
Beyond Lot Release: Stability Testing of Working Cell Banks
The shelf life of RoosterBio products are supported by long term stability studies. Stability protocols are established and executed in accordance with our overarching Product Stability Program Guideline. This procedure, shaped by ICH harmonized tripartite guidelines, outlines our internal shelf-life assignment strategy through each phase of the product lifecycle. This provides guidance on how many lots to place on stability, how frequently to test, how to continuously monitor and report data, and how to assign the final shelf-life of the product. Program management is critical here. These protocols can go out beyond five years; therefore, we ensure that proper inventory is sequestered, a testing schedule is in place, and data is monitored and reported at each timepoint to ensure products continually meet quality standards throughout their lifespan.
From Our Quality CMC to Yours: the Master File
The manufacturing and testing details of RoosterBio’s CliniControl™products are contained within RoosterBio’s Type II US FDA Biologics Master Files (MF) submitted to the US FDA for our cells and media. Maintaining up-to-date MFs for our CliniControl™products enables our customers to simply cross-reference our MFs in their US FDA submission.
RoosterBio works with customers to host quality audits, establish quality and supply agreements, and provide customer support. We invest a lot in the quality of our products with years of expertise in this field so that our customers’ can benefit from our experience. By simplifying the industry’s effort, time, and cost of individually and redundantly creating MSC banks and bioprocessing media, companies can focus on their finished product, allowing patients access to novel cell and gene therapies today rather than tomorrow.
RoosterBio is obsessed with our customers’ success. We want to see a world where safe and effective regenerative medicines are rapidly developed and are widely available on a global scale, to benefit the lives of patients in need.
