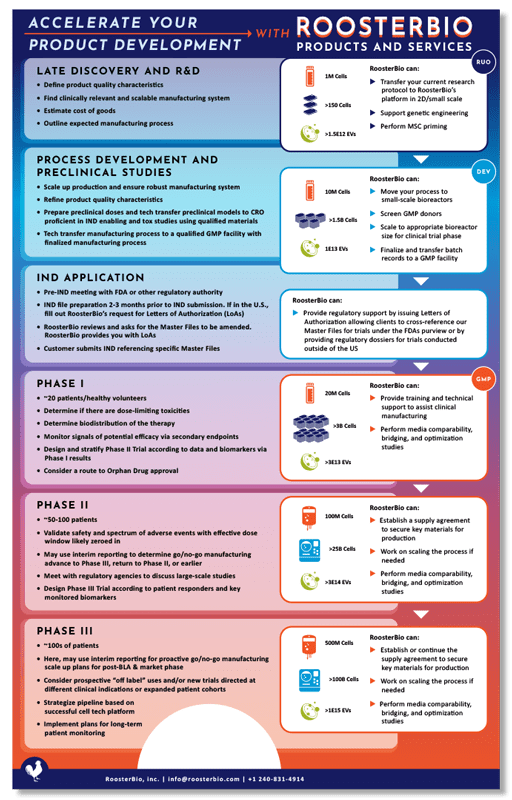
RoosterBio was founded to help mesenchymal stem/stromal cell (MSC) researchers traverse the chasm from research to the clinic. 1, 2 While that sounds all well and good, what does this mean in practical terms?
To illustrate the answer, we recently published a new infographic, Accelerate Your Product Development with RoosterBio. This blog serves as a narrative to walk you through exactly how our products and services can help, highlighting the following:
- Mission and Solutions: it illustrates how we can streamline the transition from MSC research to clinical applications by providing scalable and manufacturable MSC and extracellular vesicle platforms with an industrial supply chain of cells and media.
- Product Innovations for Process Efficiency: this combined media & cell solution is specifically engineered for bioreactor use and is available in GMP formats supported by regulatory documentation for clinical use.
- Comprehensive Support and Bioprocessing Expertise: it shows we don’t just supply products; we offer extensive support and services to help clients scale up their manufacturing processes efficiently.
Most R&D work is performed using research-grade products and processes that aren’t scalable or fit for clinical manufacturing. Thus, researchers often need to go back to the drawing board to redesign a clinically relevant process using GMP-grade cells and media—or if they have the resources, their process development group does so. 3 With every successful advancement of trial phase, this cycle is repeated as manufacturing needs grow. This constant backtracking to ensure a consistent drug product out of each new, complex process is expensive and time-consuming.
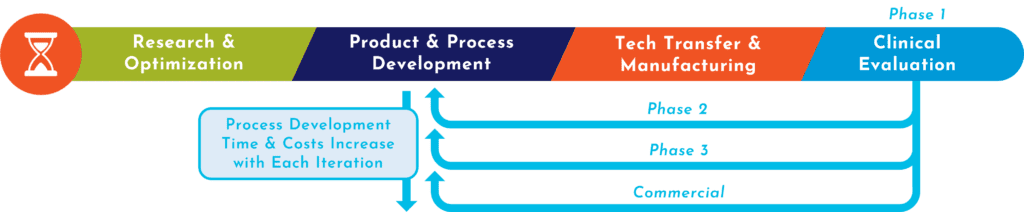
RoosterBio’s vision was to create a scalable MSC and extracellular vesicle manufacturing platform upon which researchers can plug in their IP and quickly build their products via validated, reliable, and regulatory-friendly raw materials. For the past 11 years, we’ve developed two keys to unlock quick translation:
- High-quality, high-performing products with GMP-grade analogs
- The bioprocessing know-how to manufacture MSCs within scalable systems
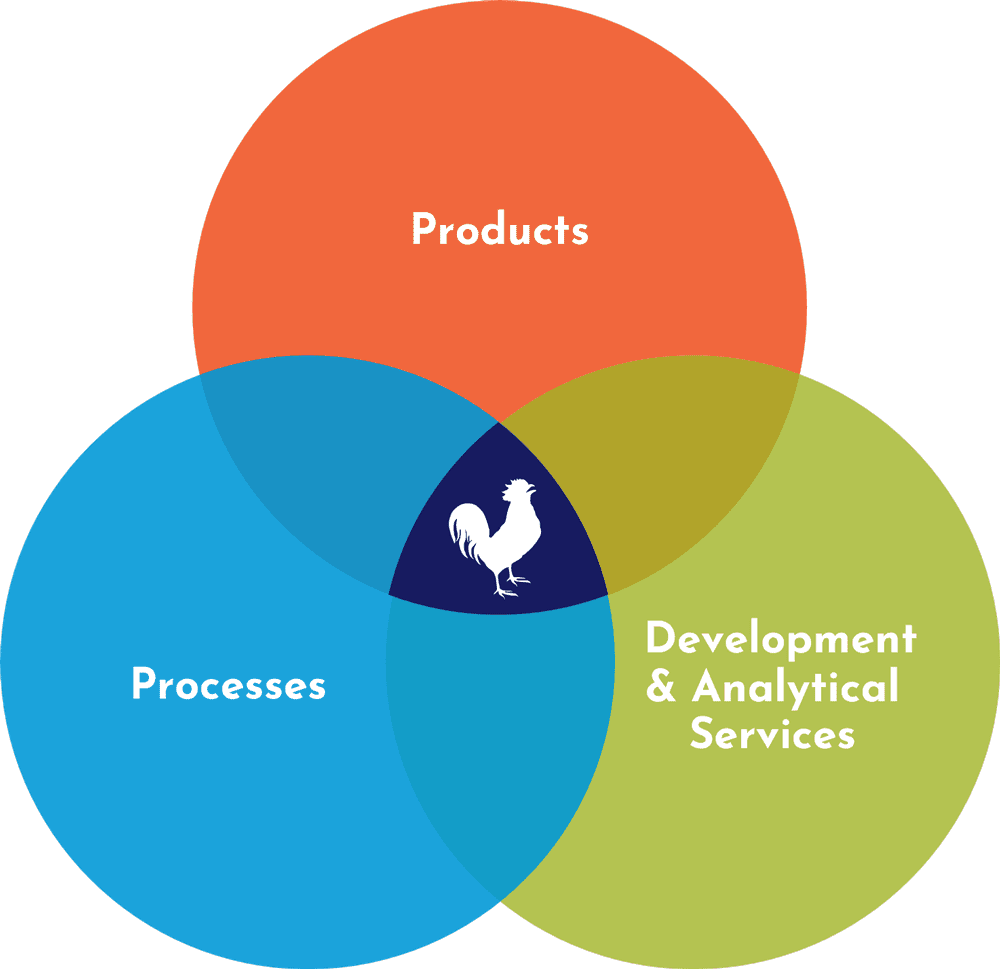
What we make—and what we do—is how we help our customers and partners move quickly. 4, 5, 6 As shown in the panels on the right of the infographic, RoosterBio is invested in the client’s success each step of the way, from the dry-erase board to the vialed doses.
Products
Our RoosterNourish™ xeno-free media expands MSCs to high numbers in 4-5 days, with no media exchange necessary. It is often the least expensive commercially available option when assessed on a per-million-cell basis due to its lack of media exchanges, unparalleled productivity, and lighter environmental footprint. 2, 7
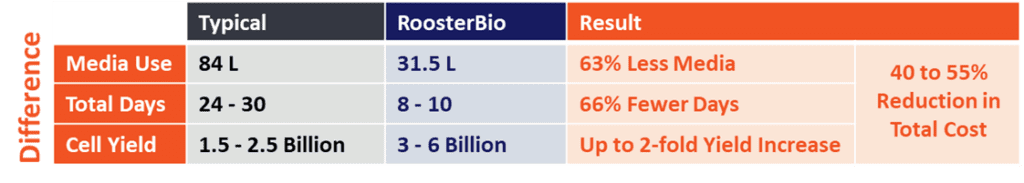
Cell expansion or conditioned media collection in bioreactors reduces the amount of medium you’ll need per million cells, which further reduces your media costs. RoosterNourish was specifically engineered for bioreactor work following virtually the same process as in flasks. With a simple feed on Day 3, you don’t need to perform large-volume media exchanges, and the time to confluence is similar. Lastly, our media are available in GMP formats and are backed by Type II Biologics Master Files with the US FDA. 8
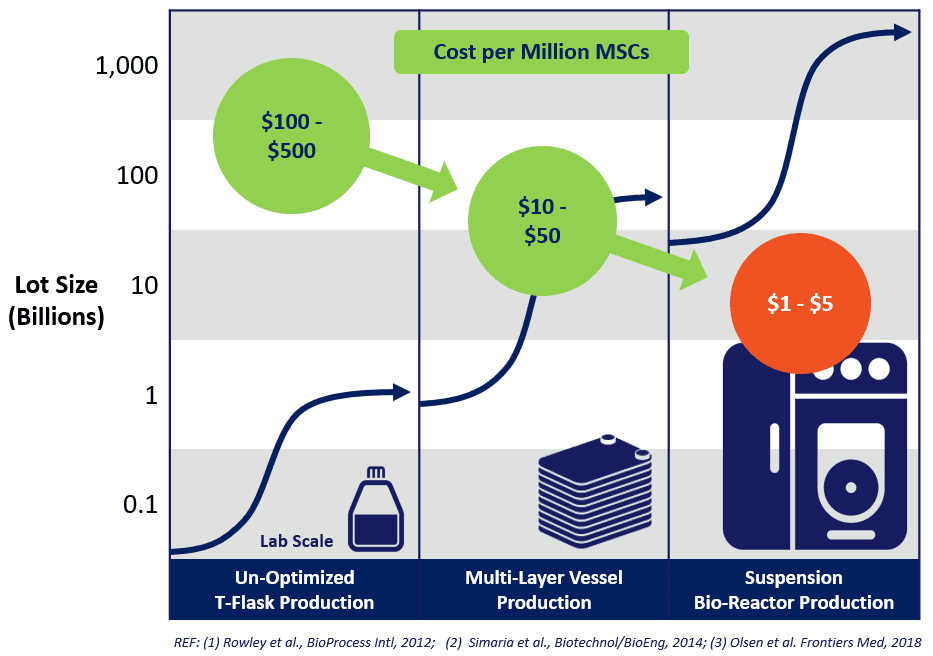
Additionally, if you don’t have access to tissue or MSC cell banks, we offer them from bone marrow, umbilical cord, and adipose tissues. These high-quality and consistent banks were created with our media and protocols and come in research, development grade, and GMP product formats for further manufacturing.
Thus, our cell and media products can grow with you from your R&D work to process development and pre-clinical studies through all clinical trial phases and commercialization.
Bioprocessing Know-How
The second critical piece for clinical translation is how to use these products within scalable bioreactor systems that can produce larger and larger batches of MSCs 9 or EVs 10, 11 with little optimization. 12 We spent many years optimizing our media systems within multiple bioreactor platforms and have built fully fleshed-out protocols for both small and large-scale MSC and EV/exosome production. 13, 14 With this expertise, we can quickly move your manufacturing process to a cost-saving bioreactor platform.

Tie Our Products & Processes Together with Unparalleled Support & Services
When you choose to work with RoosterBio products we do not let you go at it alone. 12, 15 We love supporting our customers and will work with you in any capacity you need, depending on your budget, timeline, and capabilities. We can provide a full process development services project to quickly get you to your required scale or support your own in-house development with products, protocols, and technical assistance.
Conclusion
RoosterBio has spent a decade building a path for MSC and EV translation that keeps its eye on the long-term needs of this field and removing obstacles that were traditionally major hurdles. By providing off-the-shelf, ready-to-use stem cells and a scalable media system, RoosterBio eliminates the need for time-consuming and resource-intensive isolation and expansion processes. This not only accelerates research but also significantly reduces costs, making these cutting-edge regenerative therapies more accessible to patients worldwide.
References
- Olsen, T. R.; Rowley, J. A., Corporate profile: RoosterBio, Inc. Future Medicine: 2018, Vol. 13, pp 753-757. 10.2217/rme-2018-0092
- Rowley, J. A.; Lock, L. T.; Farrance, I. K., Economics and quality attributes of hMSC production in xeno-free bioprocessing media. 2017. https://dc.engconfintl.org/cellbasedtherapies_v/50
- Campbell, A.; Brieva, T.; Raviv, L.; Rowley, J.; Niss, K.; Brandwein, H.; Oh, S.; Karnieli, O., Concise Review: Process Development Considerations for Cell Therapy. Stem Cells Transl Med 2015, 4 (10), 1155-63. 10.5966/sctm.2014-0294
- Kirian, R. Maintaining CQAs as Manufacturing Processes are Scaled from 2D to 3D Bioreactor Culture. 2022. https://www.roosterbio.com/blog/maintaining-cqas-as-manufacturing-processes-are-scaled-from-2d-to-3d-bioreactor-culture/.
- RoosterBio. On the Exosome/Extracellular Vesicle Frontier, Choose Your Own Adventure. 2023. https://www.roosterbio.com/blog/on-the-exosome-extracellular-vesicle-frontier-choose-your-own-adventure/
- Patel, A.; Candiello, J., Rapid translation of a cellular therapeutic from research to clinic. Cell and Gene Therapy Insights 2022, 08 (03), 445-445. 10.18609/cgti.2022.012
- Agbojo, O., Lim, M; Lembong, J. 2021. Environmental Analysis of Therapeutic hMSC Manufacturing: A Comparison of Multiple Bioprocess Systems. https://www.roosterbio.com/blog/environmental-analysis-of-therapeutic-hmsc-manufacturing-a-comparison-of-multiple-bioprocess-systems/.
- Williams, K.; Hansen, C. Quality Begins at Inception. 2020. https://www.roosterbio.com/blog/quality-begins-at-inception/.
- Adlerz, K.; Takacs, J.; Kirian, R. D.; Trempel, M.; Rowley, J. A.; Ahsan, T., A scalable and xeno-free bioreactor system for biomanufacturing of hUC-MSCs. Cytotherapy 2020, 22 (5), S46-S47. 10.1016/j.jcyt.2020.03.052
- Ng, K. S.; Smith, J. A.; McAteer, M. P.; Mead, B. E.; Ware, J.; Jackson, F. O.; Carter, A.; Ferreira, L.; Bure, K.; Rowley, J. A.; Reeve, B.; Brindley, D. A.; Karp, J. M., Bioprocess decision support tool for scalable manufacture of extracellular vesicles. Biotechnol Bioeng 2019, 116 (2), 307-319. 10.1002/bit.26809
- Adlerz, K.; Patel, D.; Rowley, J.; Ng, K.; Ahsan, T., Strategies for scalable manufacturing and translation of MSC-derived extracellular vesicles. Stem Cell Res 2020, 48, 101978. 10.1016/j.scr.2020.101978
- RoosterBio. Versatile Manufacturing Formats for Your Adherent Cell Therapy “Triathlon” & Its Bioprocess “Shoe Change”. 2022. https://www.roosterbio.com/blog/versatile-manufacturing-formats-for-your-adherent-cell-therapy-triathlon-its-bioprocess-shoe-change/.
- Lembong, J.; Rowley, J. Building Effective Multi-Year Process Development Programs I: Estimating hMSC Lot Size Ranges for Clinical Manufacturing Through Commercial Demand. 2021. https://www.roosterbio.com/blog/building-effective-multi-year-process-development-programs-i/.
- Lembong, J.; Rowley, J. Building Effective Multi-Year Process Development Programs II: Evolution of Technology Platform Decisions Based on Lot Size. 2021. https://www.roosterbio.com/blog/building-effective-multi-year-process-development-programs-ii-evolution-of-technology-platform-decisions-based-on-lot-size/.
- RoosterBio. “One Does Not Simply Walk Into” a Therapeutic Cell Manufacturing Process… But the Long Road Need Not Be Perilous! 2021. https://www.roosterbio.com/blog/one-does-not-simply-walk-into-a-therapeutic-cell-manufacturing-process-but-the-long-road-need-not-be-perilous/.