- The collaboration between RoosterBio and Repligen aims to address the manufacturing challenges of exosomes and extracellular vesicles (EVs), leveraging their respective expertise in primary cell-derived EV production and purification technologies.
- A joint 2023 webinar details the integrated bioprocess, combining RoosterBio’s upstream production methods with Repligen’s downstream processing technologies, to efficiently produce high-quality extracellular vesicles.
- Critical process parameters and quality attributes for extracellular vesicle production are discussed, demonstrating the effectiveness of the combined approach in maintaining extracellular vesicle integrity and bioactivity.
- Audience questions are addressed, covering topics such as cell culture conditions for extracellular vesicle generation, process scalability, and the technical aspects of EV downstream processing.
Repligen & RoosterBio Teams Double Biomanufacturing Expertise for Improved Exosome Alchemy
“Any sufficiently advanced technology is indistinguishable from magic,” Arthur C. Clarke famously quipped. While the bioproduction of therapeutic exosomes / extracellular vesicles isn’t quite magic (yet?), there’s certainly a satisfying wonderment to watch the finest biological, engineering, and professional chemistries combine to brew process innovation progress. In contrast to the “Scottish Play,” four experts from RoosterBio and Repligen portend not ill premonitions but instead, a bright common future via a joint webinar, hosted by BioProcess International.
Supply of exosomes or extracellular vesicles contends with a growing demand for their use as natural therapeutic effectors or as a cell culture-derived drug delivery system for advanced therapeutics and regenerative medicine. In the last 5 years, extracellular vesicle preparations for human doses have entered dozens of posted clinical trials, worldwide, and some are in late-Phase. Nevertheless, extracellular vesicles can be challenging to manufacture. Their inherent complexity, heterogeneity, and fragility stubbornly limit their availability for patients in need and hinder industry-wide standardization accruing to high-quality products.
Elie Zakhem, PhD, PMP, Senior Manager of Process Development of RoosterBio, kicked off this 30-minute presentation by describing the overall challenges to extracellular vesicle manufacture and how the new RoosterBio/Repligen collaboration is well-suited to solve them. Since its founding in 2013, RoosterBio has been pivotal in filling the gap in the MSC (mesenchymal stromal cell)-based product supply chain by standardizing and industrializing processes for its customers in industry and academia. RoosterBio’s scalable bioreactor processes for bulk expansion of primary adherent cells and extracellular vesicle production employ high-volume cell banks and paired high-performance xeno-free expansion media. As original pioneers of Protein A affinity systems, Repligen’s well-engineered instrument platforms and impeccable know-how to purify complex biologics from conditioned cell culture media (including viral vectors) readily translate into solutions for development and clinic-scale batches of extracellular vesicles.
With extracellular vesicles emerging on the horizon as a modality to prime Pharma pipelines for decades à la monoclonals, it was impossible for Repligen and RoosterBio to ignore one another. Thus, the collaboration’s common goal was to “deliver solutions for the manufacturing of extracellular vesicles using scalable and low shear technologies that enable cost-effective commercialization of advanced therapies.”
Dr. Elie Zakhem (RoosterBio) Spells Out a BioProcess Workflow
Timestamp 2:49
Dr. Zakhem then explained the high-level scheme for extracellular vesicle production and where each team fit (Figure 1, below).
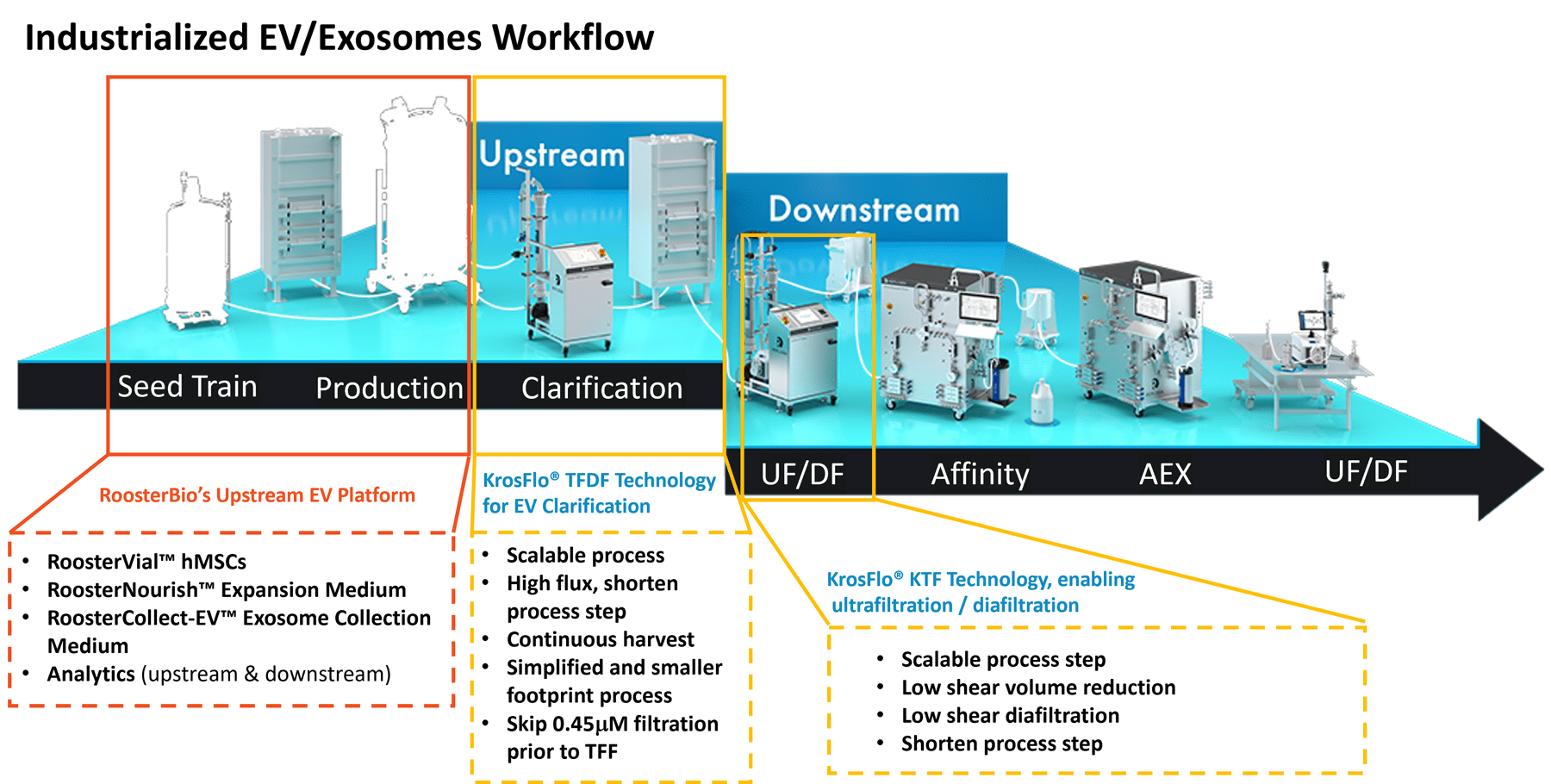
Figure 1, Combined bioprocess steps between RoosterBio and Repligen expertise domains.
Extracellular vesicle upstream production steps involved cellular seed train, cell expansion, and extracellular vesicle collection procedures using RoosterBio’s high-quality MSC media system that includes growth and EV collection media, specialized cell culture for EV production, and RoosterBio’s powerful analytical tools. Downstream processing (DSP) steps after conditioned media harvest involved clarification via Repligen’s via KrosFlo® TFDF (tangential flow depth filtration) Technology and, next, KTF ultrafiltration and diafiltration technology.
From inoculation of seed train cell material to a final 3 liters of conditioned media, the total upstream process yields a single batch preparation across 14 days (Figure 2, below).
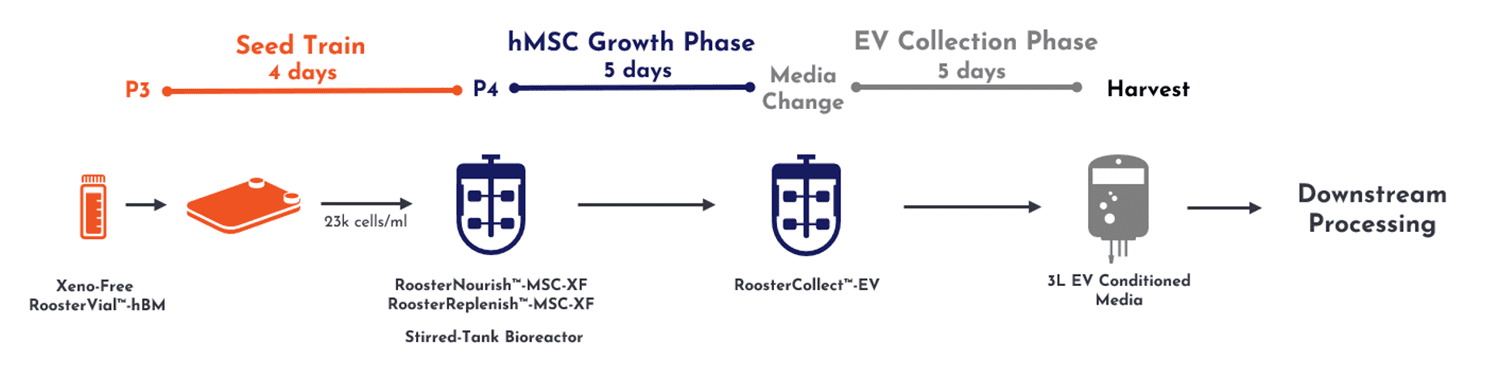
Figure 2, upstream culture of MSCs enables expansion into a 3D mini-bioreactor.
This process proceeded along critical process parameters (CPPs) developed by RoosterBio, where after 4 days of culture in 2D planar culture, cells were transferred into 3D expansion on microcarriers in a 3L stirred-tank bioreactor. After 5 days of additional growth without the need for interim media exchange, RoosterNourish™ growth medium was replaced with RoosterCollect™-EV exosome collection medium for an additional 5 days. Conditioned medium at this stage is then ready for transfer to downstream processing. This medium is manufactured to be low in particulates and not contain any non-EV contaminants from the growth media so that the extracellular vesicles are in a relatively pure state before proceeding into the downstream processing. This upstream process can be fully supported by cGMP cell and media product formats for ready, scalable translation into clinical development.
Lauren Torres (Repligen) Instructs Apprentices How Cell-Conditioned Media “Brew” is Clarified
Timestamp 6:57
After Elie Zakhem’s section, Repligen’s Lauren Torres (Field Application Scientist) took the stage to discuss Repligen’s downstream processing products and solutions of special relevance for extracellular vesicles. Specifically, KrosFlow® TFDF enables faster, scalable sterile clarification of biological samples by combining a tangential flow channel to retain large cell debris and a depth filter membrane to isolate permeate particles and macromolecules like AAV, lentivirus, advenovirus, LNPs, or EVs/exosomes. Repligen performed several phases of study to identify critical process parameters to show acceptable reproducibility and scalability (Figure 3, below).
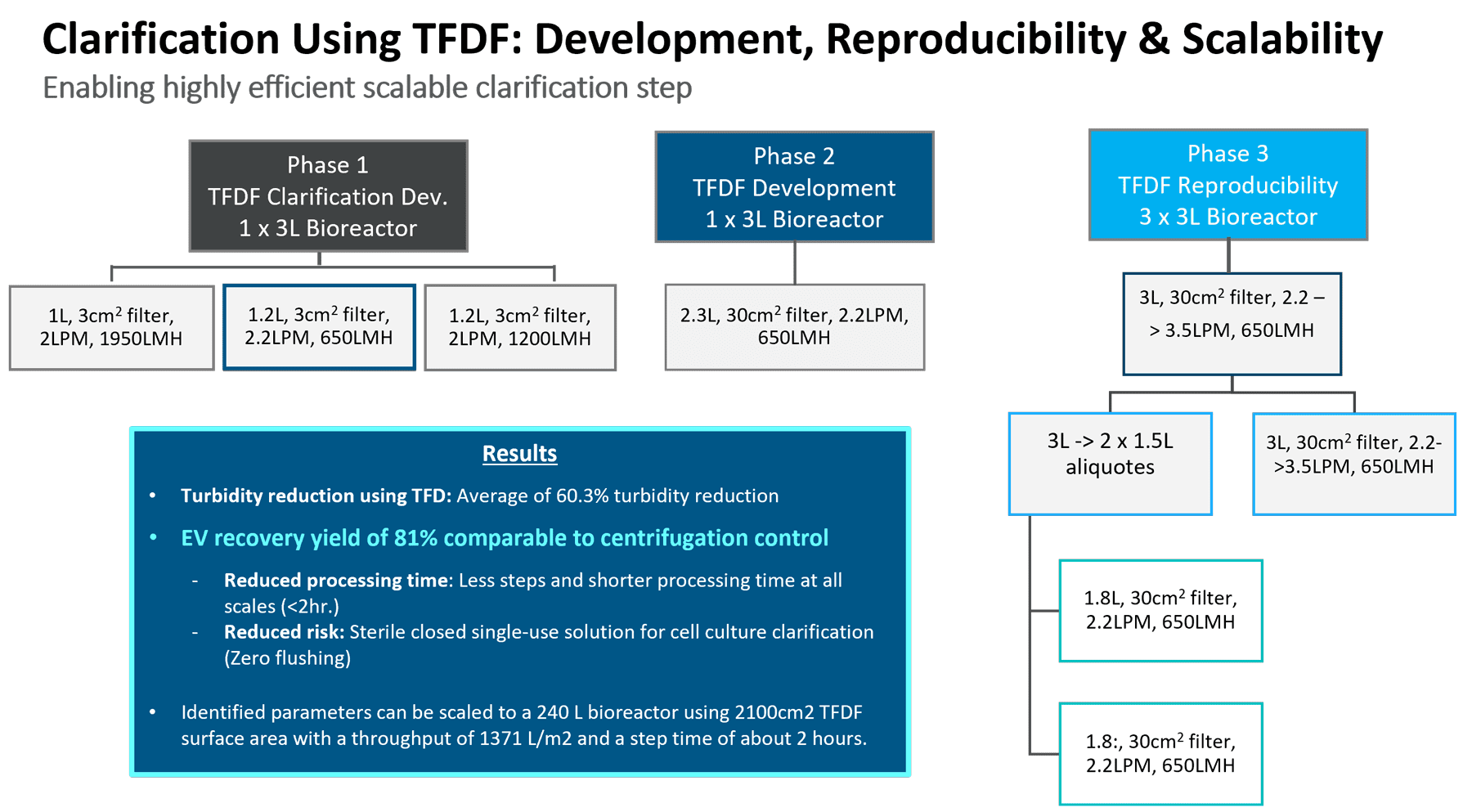
Figure 3, optimization studies for TFDF process parameters [figure resolution to be improved].
By first optimizing the flux circulation and pressure, it was next possible to scale up to a larger flow path. The last phase demonstrated process robustness and reproducibility across different processing vessels with three 3L bioreactor runs. A turbidity reduction of over 60% was achieved along with a recovery of 81% compared with the centrifugation control. Ultimately, this technology allows a reduction in processing time and risk. It should thus be reasonable to scale up to RoosterBio’s objective for a manufacturing scale of 240L volume runs, in under the two-hour processing time via the 2100 cm2 flow path (Figure 4, top table).
Jeremy Neidert (Repligen) Enchants Webinar Audience with Spellbinding TFF Data
Timestamp 10:10
Applications Scientist and Bioprocessing Account Manager, Jeremy Neidert then spoke on behalf of Repligen to explain the unique low-shear KrosFlow® KTF UF/DF (ultrafiltration/diafiltration) systems for improved product quality and recovery. Here, the intended input is the suspended TFDF clarified extracellular vesicle material, and the output is concentrated extracellular vesicles separated by a molecular weight cutoff and equilibrated in the desired buffer solution. For all studies, the KR2i TFF system was employed due to its flexible range of scales and embedded walkaway automation controls.
Phase 1 of this TFF development entailed flux excursion studies using 300-, 500-, and 750kD cutoff filters, where 750kD obtained sufficient retention and could thus permit a higher flux. Phase 2 validated at 600mL scale the empirically derived process parameters to enable their “lock-down” for future scaleup studies and applications; encouragingly brief processing times of less than 3 hours were reported. In part due to the success of the prior clarification, flux decay, and transmembrane pressure (TMP) profile was similar to most other UF/DF processes, no filter fouling was observed, and over 90% of extracellular vesicles were retained.
Now for the TFF concentration process unit scale-up in Phase 3 study! This work involved scale-up to accommodate full process batches of 3-4 liters, and generated scale-up, reproducibility, and confirmation data. From these multi-liter batch prep materials, three reproducibility runs were performed, and all resulted in less than 1% of permeate breakthrough with high recovery of EVs. Yields were approximately 4-7 trillion EVs per liter—well within the range of doses for larger animal in vivo studies.
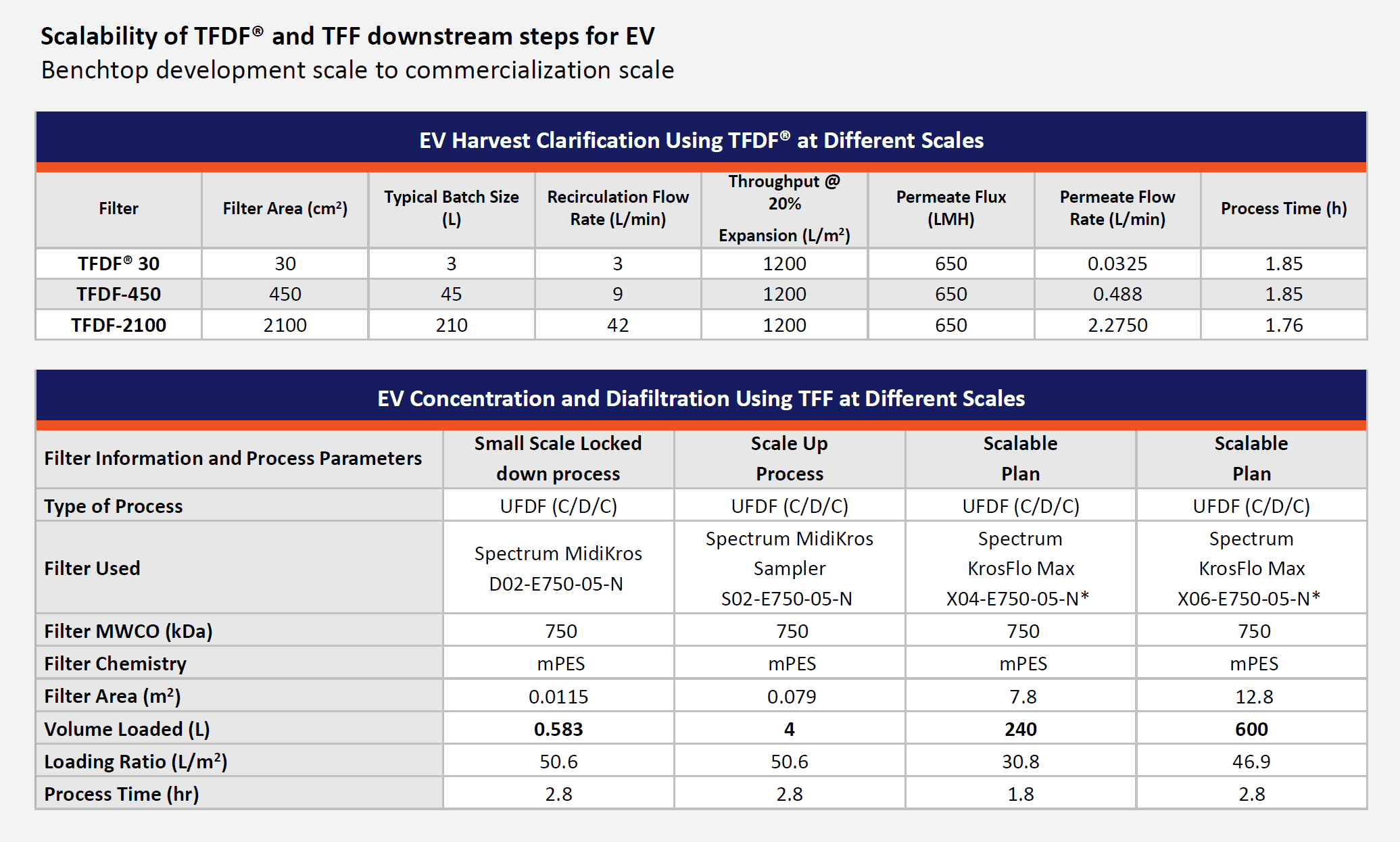
Figure 4, Top, a table showing available downstream process input volumes for TFDF clarification; this webinar featured data via the benchtop system, TFDF30®, although there are incrementally higher processing scales available. Below, a table showing experiment summaries and prospective scale-up steps via TFF instrumentation for ultrafiltration/diafiltration to concentrate extracellular vesicle samples. The lockdown parameter experiment readings are reported first, and then shown is the three experimental run summary from a Scale Up Process, followed (respectively, left to right) by prospective TFF plans to accommodate 240 or even 600 liters.
Neidert concluded his comments by summarizing the overall success of the pilot studies and reiterated how these will position a viable scale-up plan for a UF/DF instrument system (the KrosFlo Max) that could process a batch of 240 liters in less than two hours (Figure 4, Bottom table). He then returned the presentation to Dr. Zakhem for a briefing on the critical quality attributes (CQAs) obtained from the overall process.
Dr. Elie Zakhem (RoosterBio) Samples from the Exosome “Cauldron” to Test Signs of Activity
Timestamp 14:50
How did the extracellular vesicles survive the processing? Dr. Elie Zakhem described examples of extracellular vesicle analytical testing based on guidelines provided by the Minimal Information for Studies of Extracellular Vesicles group, or MISEV. These serve as some of RoosterBio’s routine assays to gather critical quality attributes that report on extracellular vesicle identity, purity, and bioactivity/potency. To validate the particles’ characteristic lipid bilayers, samples were stained with a lipid-specific dye (MemGlow™, Cytoskeleton, Inc.) and quantified by percent of particles with positive staining via ZetaView® NTA instrumentation; percent positive membrane staining did not change in TFDF or TFF output samples compared to samples from harvested conditioned media. Typical extraluminal impurities found in generation of EV-conditioned media include miscellaneous proteins, DNA, and RNA. Dr. Zakhem showed that post-processing treatments exhibited over 50% clearance relative to extracellular vesicles fresh from conditioned media.
Identity of the particles was further confirmed by Western blot analysis of samples from three stages of purification: (1) harvested conditioned media, (2) TFDF® clarification, and (3) TFF concentration. Canonical extracellular vesicle/exosome tetraspanins markers (CD9, CD63, CD81) were all identified with signs of enrichment through the processing steps. Furthermore, sample EVs following downstream processing steps showed signs of retained biological activity by their effect on a cell wounding assay in which there is image quantifiable repopulation and/or migration into a linear “scratch” marked across a well of confluent cells (Figure 5).
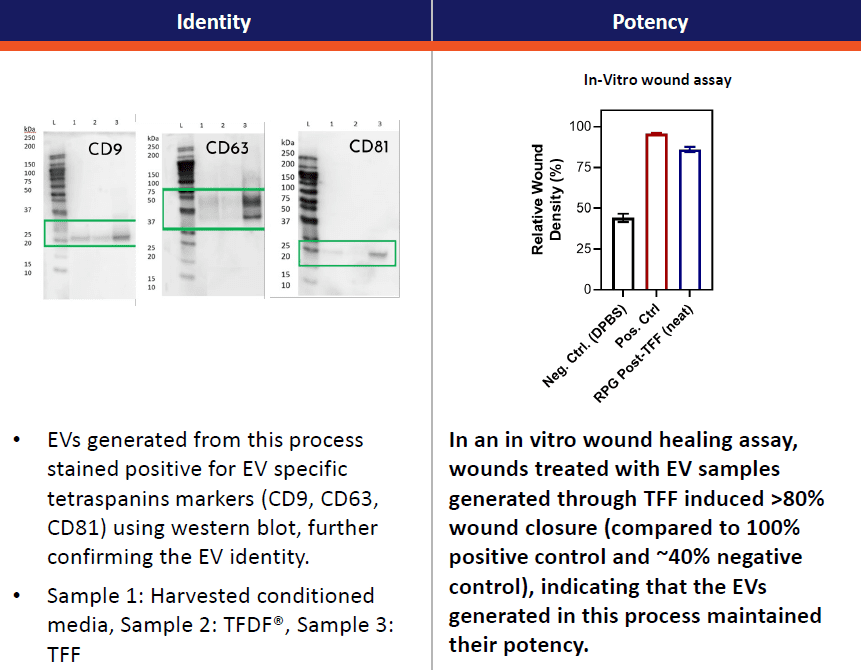
Figure 5, left, representative Western blots of canonical extracellular vesicle/exosome markers (CD9, CD63, and CD81) showing apparent enrichment in concentration across DSP steps of (Lane 1, initial harvest of conditioned media; Lane 2, samples after TFDF clarification; and Lane 3, samples after harvest, TFDF, and TFF). Right, experiment showing enhanced cell culture wound closure assay performance via the addition of bioprocessed extracellular vesicles/exosomes.
Zakhem then concluded the webinar at this juncture and then yielded the floor to a lively session of Q&A. Stephen Lenzini, PhD, Process Development Scientist at RoosterBio, stepped in to assist with the answers, along with Repligen’s Lauren Torres and Jeremy Neidert.
An Inquisitory Webinar Conclusion
Timestamp 20:30
Some of the audience questions are listed below:
- Were the cells preconditioned before generating extracellular vesicles?
- Are you using any kind of permeate flux control on your final TFF set?
- Was biocompatibility performed on custom consumables per ISO 10993? If so, how extensive was it? I.e., how many parts of the standard were tested to?
- What is the extracellular vesicle storage buffer in temperature for long-term storage?
- Can you speak to TFDF membrane’s lifetime or fouling?
- Is impurities clearance not quite yet efficient? Does your process meet the criteria for purity? What is the remaining protein impurity?
- Do you recommend using the 750 KDa more than the 500 KDa cut off to recover extracellular vesicles? Do you have comparisons of size distribution for recovery between both filtration?
- Did you do a single collection of extracellular vesicles after five days in RoosterCollect and did you monitor nutrient consumption?
- Where do you prepare the source of your primary cells and where are they isolated and processed for commercialization?
- What is your intended use of extracellular vesicles clinically? For example by IV injection, IM, or any other route?
It’s far better to absorb the answers to these questions by logging into the webinar recording at BioProcess International. So why don’t you give this 30-minute presentation a view? RoosterBio is most proud of this collaboration with the illustrious Repligen, and greatly looks forward to our next steps to bioproduce even larger preps from our extracellular vesicle/exosome “cauldrons.”
