RoosterRTP™-hBM-50M-XF
Ready-to-Print Formulated Xeno-Free Human Bone Marrow-Derived Mesenchymal Stem/Stromal Cells
Fully-expanded, translation-friendly hMSCs – ready for same-day experiments.
Recommended Products
Overview
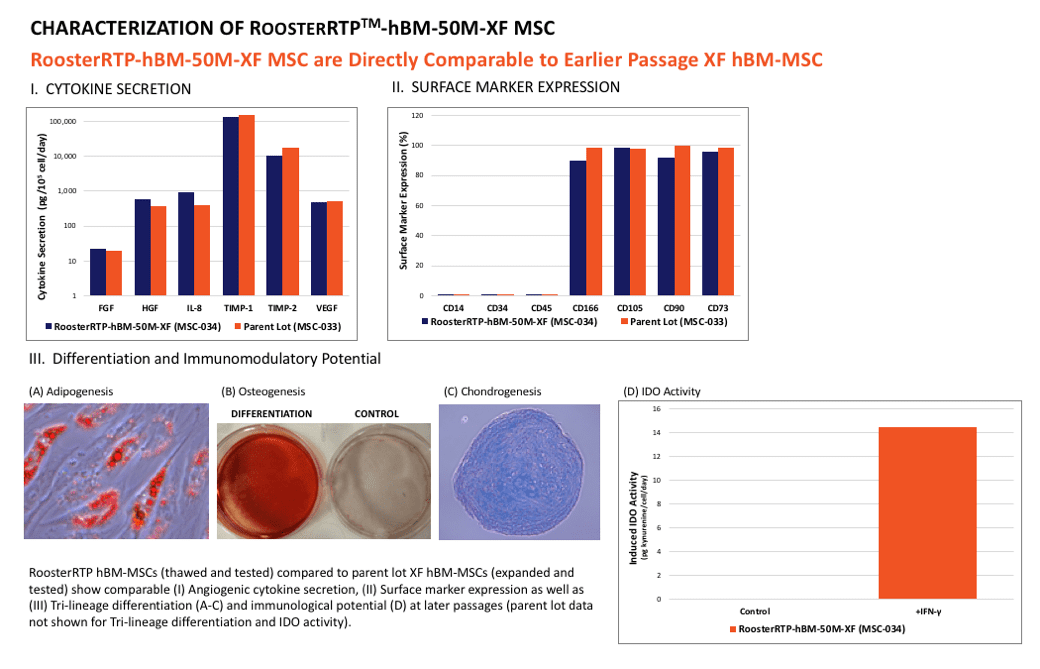
Ready-to-print and ready-to-seed into your biofabrication application. RoosterRTP™-hBM-50M-XF are xeno-free formulated (XFF) late passage hBM-MSCs that are translation-friendly, clinically-relevant, and intended to be thawed and used in a research setting. They are not intended for further expansion or banking. RoosterRTP-hBM-50M-XF are expanded using controlled manufacturing processes from a Master Cell Bank for 4 passages to a PDL of 15-17, and then harvested, formulated, filled, and cryopreserved. As reported in the accompanying QC Brief, these cells maintain the critical MSC quality attributes at late-passage of the parental cell lot (cell surface marker expression, trilineage differentiation, immunomodulatory potential (IDO assay), and angiogenic cytokine secretion). This ready-to-use critical raw material enables product developers and researchers to forgo the cell manufacturing process, skipping cell culture altogether.
Product Features
- 50 million late-passage (PDL 15-17) cryopreserved hBM-MSCs
- Xeno-free formulation (XFF)
- No culture required. Not intended for further subculturing and banking.
- Minimize time between experiments for accelerated discovery
- Rapidly prototype tissue designs
- Add as the cell component of bioink
- Seed directly into tissue engineered scaffolds, or incorporate within a medical device
- Readily study biomaterial and biofabrication effects on tri-lineage differentiation, cytokine secretion profile, and inducible immunomodulatory function
- Industry-leading functional characterization
- Preserved critical MSC quality attributes at late-passage
- Comparable to parent lot
- Manufactured with scalable processes & cryopreserved using standard, controlled processes
- Lots released based on viability, plating efficiency, and sterility
Intended Use: Research Use Only
Protocols and Information
Login to search Certificates of Analysis and Quality Control Briefs.