Listen to this Blog:
“It’s a dangerous business, Frodo, going out your door. You step onto the road, and if you don’t keep your feet, there’s no knowing where you might be swept off to.”
– J.R.R. Tolkien
As Tolkien’s fictional character, Bilbo Baggins, told his nephew, Frodo, some tasks are not for the faint of heart, and everything from epic journeys to cell & gene therapies [1, 2, 3, 4] need some first steps. Yet, even before taking them, it’s prudent to reflect on the journey ahead. RoosterBio’s domain expertise in bioprocess development for cell therapy manufacturing can help.[5, 6, 7]
Human regenerative medicine (“regenmed”) therapeutics that rely on ex vivo propagation of primary cells are complex.[8, 9] First, one must sift through a series of candidate discovery materials + bioprocess combinations to obtain a lead with compelling in vivo activity.[10, 11, 12] Then, a cell manufacturing process [13] must consistently yield biomaterial with the desired attributes, maintaining chain-of-identity through a series of handoffs under close monitoring and tracking.[14, 15] The clinical manufacturing plan must right-size the scale required for each step in the trials process—and yet also not fatally modify this process to a degree that it’s ruled to be a separate product.[16, 17]
Sound daunting? Take courage! In March 2021, RoosterBio published a well-received duet of blog articles to answer your questions about how to get started. The first blog helps answer how to estimate the number of cells you will need to make, as each development step ratchets up in volume scale.[18] The second blog helps you zero in on which technologies can best align with both your needs at a given development stage, from “upstream” (working cell banks and cell expansion) to “downstream” (harvest, volume reduction, formulation, and cryopreservation).[19] In this “mini-blog,” we summarize both articles and provide tools and diagrams to help you take these first steps, while cognizant of the whole process.
In the Part 1 Blog (Estimating hMSC Lot Size Ranges for Clinical Manufacturing Through Commercial Demand), authors Josephine Lembong, Ph.D., and Jon Rowley, Ph.D., helped outline a development program that could tailor accordingly to meet your final product platform needs, with target cell lot size foremost in mind. First, “white-board” the plan’s overall shape like this:
- Understand what the future looks like and work backward
- Create a model using reasonable conservative assumptions along the way to estimate a range of needs
- Use this model to lay out how the next several years will play out, assuming successful achievement of intermediate milestones
- Create multi-year programs that are right-sized, with the right technology platform, for the stage of product/clinical development of your company
To strategically align manufacturing requirements today—with commercial scalability—calculate the future lot size requirements and lay out a platform foundation to minimize comparability risks. “Estimating hMSC Lot Size Ranges” (Part 1)[18] provides a two-step thought process in their diagram:
- Calculate yearly product needs at peak commercial demand; AND
- Incorporate crucial assumptions during every step of the manufacturing process to calculate all cells required to meet above need
Linked below in this blog is an online spreadsheet tool you can use to walk through and toggle those basic, pivotal assumptions. It can be applied to final product at its peak demand—but it could also be used to imagine earlier steps at smaller scale during the manufacture of trial material.
Calculate Lot Sizes for Your Trials or Commercial Manufacturing
Primary cells like human mesenchymal stromal/stem cells (hMSCs) could have utility for a vast range of human medical conditions, some of which involve 100s of thousands of patients annually.[4, 20] Early on, the first draft of a Target Product Prolife (TPP)[21] is crucial for honing the specs of the cellular drug product, and precisely whom it’s intended to treat, and, also, for which indication. (Recall that each indication is regarded as a unique product with its unique manufacturing process.) Lembong and Rowley posited a scenario here where sufficient material for 100,000 patients is ultimately required in the peak year, translating to more than 12 trillion cells manufactured across 23 manufacturing lots. Meeting this demand today would require an ambitious leap in dedicated cell manufacturing CDMO capacity. Thankfully, the basic process technology units of this production are known (see Part 2 blog), and can be extrapolated on in the event of a successful Agency approval. As Lembong and Rowley noted, “It is important to remember that it typically takes multiple years for a successful therapeutic to reach peak demand, so it is not necessary to go to market with a manufacturing process capable of meeting peak demand – especially for an early-stage field like Regenerative Medicine.”[18] Thus, this article first creates estimates for peak demand, but then focuses on building a reasonable “go-to-market” lot size and manufacturing process. They estimate that for this hypothetical hMSC therapeutic, numbers during clinical trial phases are easier to grasp:
- Phase I (small scale): 5-10B cells
- Phase II (100-200 patients over 3-4 lots): 15-25B cells – this is an intermediate step up from Phase I and within a log of the Phase III/go-to-market process
- Phase III/Go-to-Market: ~100B cells
For your own practical purposes, Lembong and Rowley emphasized that appropriate cells per dose calculation is not the final assumption. One must also account for all the potential losses along the way in a manufacturing run, which often range from 40%-60% in downstream processing (DSP) in biologicals manufacture. Also, one needs to consider the drop in number of viable cells and recovery losses post-thaw, such that doses are planned for a level of container vial overfill. Also, let’s not forget that a fraction of material will go not into dosing but rather into lot testing, and that occasionally, some manufacturing runs just fail.
In the Part 2 blog article (Evolution of Technology Platform Decisions Based on Lot Size),[19] our authors help you navigate the large “garage” of bioprocess tools for their best fitting tasks. In terms of technology platforms, one challenge is to maintain focus for the shot on goal, but also keep its process sufficiently flexible and scalable for additional applications in the future. Thus, they recommend a “2D expansion” process for the Phase I trial to yield ~10B cells of trial cellular material via stacked flasks. Then, for Phase II, it will likely be appropriate to migrate into a more-efficient “3D expansion” mode (w/ microcarriers) to reach the target of 25B cells; Phase II is where it becomes labor- and time-intensive to juggle ~100 CellStacks, not to mention environmentally unfriendly. For Phase III and thereafter, 3D is all but essential to reach cell volumes sufficient for the patients in need.
By way of the Evolution of Technology Platform blog,[19] the authors explained their rationale in much greater detail, but it’s helpful that they generalized some of their thought process with this Table below:
Summary of the Technology Landscape for Allogeneic hMSC Manufacturing – by Unit Operation
|
Stage |
Lot Size | Working Cell Bank | Culture Platform | Volume Reduce / Wash | Fill / Finish |
Cryopreservation |
| Phase I | 10B | 40M cells in vials / bags | 2D (40 x CS10) | Open or continuous centrifugation | Semi-auto vials, 250/hr | Controlled-rate freezer |
| Phase II | 25B | 100M cells in vials / bags | 3D (50-80 L) | Open or continuous centrifugation | Semi-auto vials, 250/hr | Controlled-rate freezer |
| Phase III / Go-to-market | 100B | 400M cells in bags | 3D (200 L) | Continuous centrifugation | Automated vial line 500+/hr | Controlled-rate freezer |
| Full commercial scale | 500B | 2B cells in bags | 3D (1000 L) | Large scale continuous centrifugation | Fully automated vial line 3000+/hr | Scaled-up controlled-rate freezer |
Here, you can see that different technology platforms are good for different scales. Phase I (and earlier) trial materials don’t necessarily require up-front investment in bioreactor equipment and could be carried out by a fairly small scale operation with reagents, practices, and settings that are familiar to most academic cell researchers. In time for Phase II, investment in “3D” involves some learning curve, but this transition becomes viable due to large gains in cell and media productivity and efficiency. Ultimately, a large-scale manufacturing lot for an approved clinical product could need 1000-liter bioreactors for each lot run, with accompanying working cell banks numbering up to 2 billion cells. To reduce human error, the level of automation increases throughout the phases.
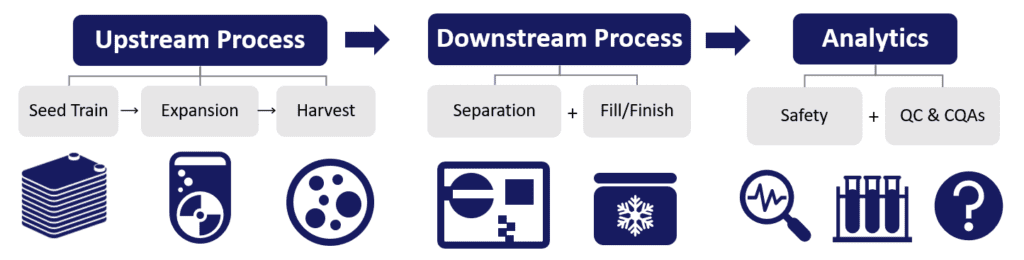
How do we reach these scales? In the above diagram, basic component elements can stretch across the same basic process, irrespective of scale. By evolution of the platform tool use across this template, it can be possible to obtain cellular products of comparable consistency across quality attributes. Authors Lembong and Rowley appropriately described in Part 2 some validated practices and product systems that—when used in concert—accrue to unique advantage over “DIY” (“do it yourself”) approaches that start from scratch. If interested, this material is well-worth your read!
They concluded:
“We have highlighted various existing technology platforms involved in an hMSC manufacturing process. You can think of this exercise as a decision-making tool to help streamline an hMSC clinical manufacturing program. As this tool informs the development team on the unit operation choices associated with each phase, it also helps the team define their requirements and invest in lot-size-appropriate technologies. This leads to a RegenMed company with a clinical pipeline to plan for a multi-year process development program to meet the peak commercial demands for most indications that would demand hMSC treatment.”
To paraphrase an-often recycled meme from The Lord of the Rings, “One does not simply walk into” Cellular Therapy Bioprocess. Nevertheless, the journey no longer needs to be fraught with unknown hazards, and there can indeed be a clear path to reach your destination.
If you have an interest in discussing scalable production platforms, our complete solutions that radically simplify hMSC process development or our Process Development Services, please reach out to us at info@roosterbio.com!
References
- Abernathy, William J. and James M. Utterback, Patterns of industrial innovation. Technology review, 1978. 80(7): p. 40-47.
- Carlson, Robert H., Biology Is Technology: The promise, peril, and new business of engineering life. 2011, Harvard University Press. ISBN-10.
- Olsen, T. R. and J. A. Rowley, Corporate profile: RoosterBio, Inc. Regen Med, 2018. 13(7): p. 753-757. 10.2217/rme-2018-0092
- Olsen, T. R., et al., Peak MSC-Are We There Yet? Front Med (Lausanne), 2018. 5: p. 178. 10.3389/fmed.2018.00178
- Lembong, Josephine, et al., A scalable xeno-free microcarrier suspension bioreactor system for regenerative medicine biomanufacturing of hMSCs. 2019.
- Takacs, Joseph; Adlerz, Katrina. hUC-MSC Exhibit Robust Proliferation in 3D Bioreactor System. RoosterBio Blog 2020; Available from: https://www.roosterbio.com/cell-therapy/huc-msc-exhibit-robust-proliferation-in-3d-bioreactor-system/.
- Lembong, J., et al., Bioreactor Parameters for Microcarrier-Based Human MSC Expansion under Xeno-Free Conditions in a Vertical-Wheel System. Bioengineering (Basel), 2020. 7(3). 10.3390/bioengineering7030073
- Witcher, Mark. Phase III Clinical Trials – Ever Wonder Why Some Products Unexpectedly Fail? Pharmaceutical Engineering 2019; Available from: https://ispe.org/pharmaceutical-engineering/ispeak/phase-iii-clinical-trials-ever-wonder-why-some-products-unexpectedly-fail.
- de Almeida Fuzeta, M., et al., Addressing the Manufacturing Challenges of Cell-Based Therapies. Adv Biochem Eng Biotechnol, 2020. 171: p. 225-278. 10.1007/10_2019_118
- Ahmed, Y., et al., Impact of combined therapy of mesenchymal stem cells and sitagliptin on a metabolic syndrome rat model. J Diabetes Metab Disord, 2021. 20(1): p. 551-560. 10.1007/s40200-021-00778-3
- Bonafont, J., et al., Correction of recessive dystrophic epidermolysis bullosa by homology-directed repair-mediated genome editing. Mol Ther, 2021. 29(6): p. 2008-2018. 10.1016/j.ymthe.2021.02.019
- Mansouri, M., et al., Smart-watch-programmed green-light-operated percutaneous control of therapeutic transgenes. Nat Commun, 2021. 12(1): p. 3388. 10.1038/s41467-021-23572-4
- Rowley, Jon, et al., Meeting lot-size challenges of manufacturing adherent cells for therapy. BioProcess Int, 2012. 10(3): p. 7.
- Jayaraman, P., et al., Acceleration of Translational Mesenchymal Stromal Cell Therapy Through Consistent Quality GMP Manufacturing. Front Cell Dev Biol, 2021. 9: p. 648472. 10.3389/fcell.2021.648472
- Williams, Kathy; Hansen, Caitlin. Quality Begins at Inception. 2020; Available from: https://www.roosterbio.com/blog/quality-begins-at-inception/.
- (FDA), US Food and Drug Administration. Cellular & Gene Therapy Products. FDA.gov 2021; Available from: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products.
- Lim, Mayasari. Know Your Cost of Goods Recap and Managing Lot Size Estimation. RoosterBio Blog 2020; Available from: https://www.roosterbio.com/blog/know-your-cost-of-goods-recap-and-managing-lot-size-estimation/.
- Lembong, Josephine; Rowley, Jon. Building Effective Multi-Year Process Development Programs I: Estimating hMSC Lot Size Ranges for Clinical Manufacturing Through Commercial Demand. RoosterBio Blog 2021; Available from: https://www.roosterbio.com/blog/building-effective-multi-year-process-development-programs-i/.
- Lembong, Josephine; Rowley, Jon. Building Effective Multi-Year Process Development Programs II: Evolution of Technology Platform Decisions Based on Lot Size. RoosterBio Blog 2021; Available from: https://www.roosterbio.com/blog/building-effective-multi-year-process-development-programs-ii-evolution-of-technology-platform-decisions-based-on-lot-size/.
- Caplan, A. I. and D. Correa, The MSC: an injury drugstore. Cell Stem Cell, 2011. 9(1): p. 11-5. 10.1016/j.stem.2011.06.008
- Campbell, A., et al., Concise Review: Process Development Considerations for Cell Therapy. Stem Cells Transl Med, 2015. 4(10): p. 1155-63. 10.5966/sctm.2014-0294
