What does an elephant have in common with a bioreactor? The answer’s in the ears. While our majestic mammalian friend uses her large ears to stay cool and communicate better, Satorius Stedim Biotech (SSB) recently innovated an “elephant ear” impeller into its Ambr®250 High Throughput bioreactor vessels to reduce shear stress for adherent cell culture on microcarriers or T cells on activating beads. [1] Not only has this Ambr® configuration proven useful for vaccine process development optimization, we and others are also demonstrating that it’s remarkably versatile towards human mesenchymal stromal/stem cells (hMSCs) and their exosome/extracellular vesicle (EV) secretomes.
The Ambr’s® secret of success is that it enables plug-and-play adaptation towards diverse bioproduction schemes from E. coli to Pichia to CHO, yielding comparable performance in cell expansion and protein production at much higher scales of 50 to >1000L. Like the Ambr®250, hMSCs are also very engineerable in their own way. Easy to grow and administered safely across thousands of clinical trials—and in several marketed cell therapies beyond the USA—each hMSC-based therapeutic must nevertheless still prove that its underlying process will yield a reliable product. That is, your group’s fastidious optimization of process parameters at small scale may involve cell-media pairing, donor- or tissue-specific GMP master cell banks, and physical/mechanical settings. Yet one day, the process will likely incorporate changes for much larger scales. [2]
In one trial aimed at the 65 million worldwide sufferers of COPD, for example, 1.6 billion hMSCs per patient were injected; a hypothetical commercial cellular drug to assist only 65,000 such patients could thus demand over 100 trillion cells! [3] Thus, to justify the time and expense of “going large” on a fully baked-in bioprocess, early pivotal decisions must be thoroughly de-risked and modeled at small scale, given that productivity doesn’t always linearly correlate with volume. To advance beyond this dilemma, we’ve found that partnerships and collaborations with organizations like SSB are mutually beneficial, where Sartorius contributes its vast hardware expertise via the bioreactor platforms, microcarriers, DOE methodologies, and data analytics toward rapidly engineered, fit-for-purpose solutions. [4] In turn, we employ our co-optimized combination of cell and media products, bioprocess development expertise, and analytical services that are specialized for hMSCs and MSC-EVs, but functional across wide-ranging bioreactor and planar “2D” systems. Together, our joint solution credibly speaks to production runs that would be sufficient to supply Phase II or Phase III trials for hMSCS or hMSC-EVs/exosomes.
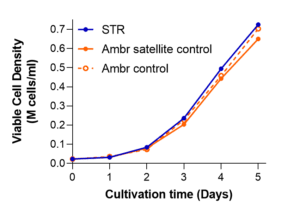
How does that look in real life? Recently, we presented data at the 2022 International Society for Extracellular Vesicles (ISEV) Meeting in Lyon, France. [5] Compared with culturing in Ambr250® vessels, scaling up to a 15-liter “pilot” volume via the Sartorius STR® 50L scale bioreactor closely followed expansion kinetics observed with the 0.25L volumes. We reported similar final densities exceeding ~500K cells per ml, in similar 5-day time spans—in hMSCs from bone marrow or from umbilical cord (Figure 1, below).
| Bone Marrow MSC | Umbilical Cord MSC |
|---|---|
 |
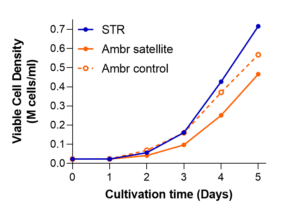 |
Figure 1. A media-exchange-free MSC STR® microcarrier expansion process with bone marrow-derived (hBM, left) and umbilical cord sourced (hUC, right) hMSCs is scalable at 15L. These representative data were verified with multiple donors’ cell banks.
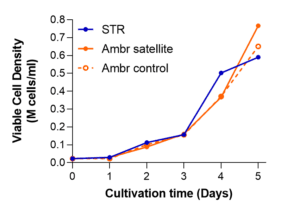
Running at “full scale,” 50L production volume with the STR®, the process was validated in two separate runs, yielding more than 25 billion cells per lot (Figure 2, below). The 5-day process was again closely modeled with Ambr®250 vessels, reaching similar densities as at large scale. As before, due to the optimized nutrient and growth factor formulation, our process did not require an exchange of media after the initial cell seeding.
 |
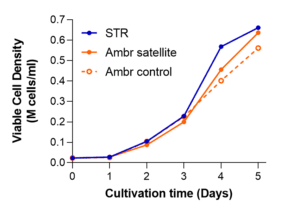 |
Figure 2. A media-exchange-free MSC STR® microcarrier expansion process with bone marrow-derived hMSCs is similarly scalable at 50L, compared with Ambr® vessels, across two separate runs (left and right).
Presentation of these data at ISEV-2022 underscores hMSCs’ prominent role in exosome/extracellular vesicle bioproduction for more than half of related clinical trials, [6] and it affirms the scalability of a classic stirred tank configuration that could one day accommodate volumes of up to 2000L per run. Ambr® High Throughput vessels could likewise be initially applied to different R&D candidates of molecularly cargoed or targeted exosomes, permitting a more comfortable handshake for “engineerability” between the nanoscale via proteins/miRNAs and the macro-scale with cell metabolism/media. For these extracellular vesicle therapeutics to consistently demonstrate their critical quality attributes, however, their parent cells must reliably retain the hMSC identity and phenotype. We examined this question too, comparing a “traditional” 2D bioprocess on tissue culture plastic versus the Sartorius process with microcarriers. As shown in Figure 3 (below), the surface marker profile, immunomodulatory activity with IDO (indoleamine 2, 3-dioxygenase), angiogenic factor panel expression, and trilineage differentiation to bone, adipocytes, or cartilage were all reassuringly the same between 2D and the “3D” SSB processes.
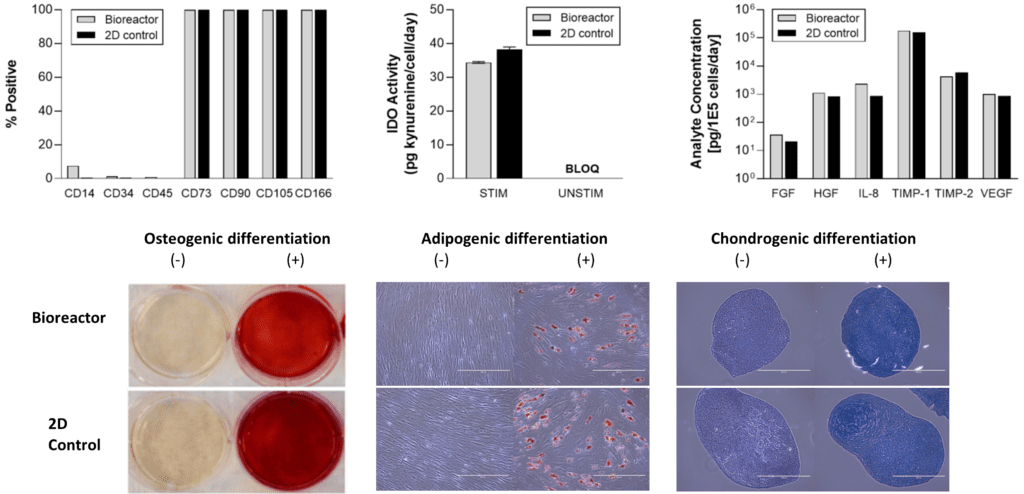
Figure 3. Harvested hMSC population was highly comparable in both identity and functional attributes to cells grown under traditional 2D culture conditions.
What next? Average human doses for an exosome/extracellular vesicle therapeutic (widely ranging with injection route and indication) are approximately 4×1011EVs/dose. Given our methods where EVs can be collected in yields of more than 1010/ml, the 50L STR® format could potentially yield thousands of doses for advanced phase trials and beyond, assuming downstream process (DSP) efficiency reaches a state-of-the-art. [7] After that, one can’t help but wonder what a 2000L bioreactor could do. In the meantime, smaller volumes from multiple scalable Ambr® system vessels could help drive to a more rapid optimization of conditions for both upstream and downstream EV preparations.
Who knew that elephant ears evolved into their current form, in part, under strong selection pressure to communicate via infrasound calls across many kilometers of open savannah? By analogy, the “elephant ear” impeller design for stirred tanks also went through its own “evolutionary” trial and error. To best manage the bioreactor’s unpredictable fluid dynamic effects on microcarrier cell expansion, perhaps it helps to look to nature for inspiration? Of course, as we all gather around the “watering hole” in anticipation of biotechnology’s next rainy season, it’s worth concluding that we consider it a great privilege to mingle with our fellow creatures in the biz, great and small!
References
- Rotondi, M., et al., Design and development of a new ambr250(R) bioreactor vessel for improved cell and gene therapy applications. Biotechnol Lett, 2021. 43(5): p. 1103-1116. 10.1007/s10529-021-03076-3
- Olsen, T. R., et al., Peak MSC-Are We There Yet? Front Med (Lausanne), 2018. 5: p. 178. 10.3389/fmed.2018.00178
- Mesoblast, Inc and Ltd Mesoblast, PROCHYMAL™ (Human Adult Stem Cells) for the Treatment of Moderate to Severe Chronic Obstructive Pulmonary Disease (COPD). 2009.
- Satorius. Sartorius Partners With RoosterBio to Advance Cell and Gene Therapy Manufacturing. 2021; Available from: https://www.sartorius.com/en/company/newsroom/corporate-news/sartorius-partners-with-roosterbio-to-advance-cell-and-gene-therapy-manufacturing-640284.
- RoosterBio. RoosterBio Joins the Action at ISCT, ASGCT, & ISEV in May 2022. RoosterBio Blog 2022; Available from: https://www.roosterbio.com/blog/roosterbio-joins-the-action-at-isct-asgct-isev-in-may-2022/.
- Silva Couto, Pedro. Exosome Clinical Trials. celltrials.org 2022; Available from: https://celltrials.org/public-cells-data/subscribe-to-exosome-clinical-trials/80.
- Jung, Jai and Lenzini, Stephen. Extracellular Vesicle/Exosome Downstream Process Development Part I: Leveraging Filtration Technologies for Scalable EV Preparation. RoosterBio Blog 2022; Available from: https://www.roosterbio.com/blog/extracellular-vesicle-exosome-downstream-process-development-part-i-leveraging-filtration-technologies-for-scalable-ev-preparation/.
