Listen to this Blog:
Fibrosis: A Serious Unmet Medical Need
Fibrosis—commonly known as scarring—can exact a heavy toll on sufferers. In the developed world, a surprising 45% of all mortality is driven by severe fibroproliferative disease processes of the inner organs and circulatory system. [1, 2, 3] These include common atherosclerosis, but also idiopathic pulmonary fibrosis (IPF), liver and kidney fibrosis, systemic sclerosis, and abdominal adhesions. Approved medications to treat fibrosis directly remain of limited efficacy, only relieving symptoms. With the exceptions of nintedanib and pirfenidone for IPF, [4] these drugs usually don’t directly antagonize the molecular drivers of this disease class as we currently understand it.
Figure 1A
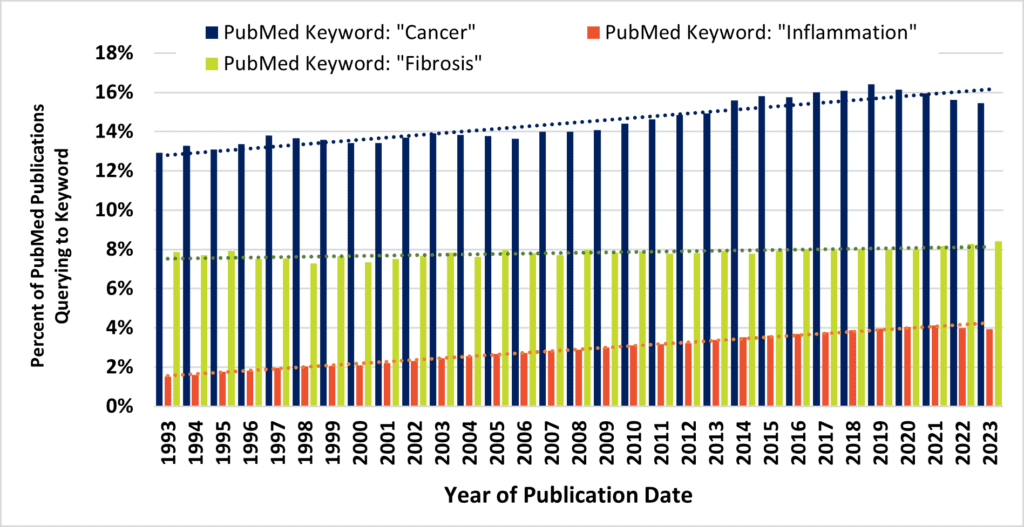
Figure 1B
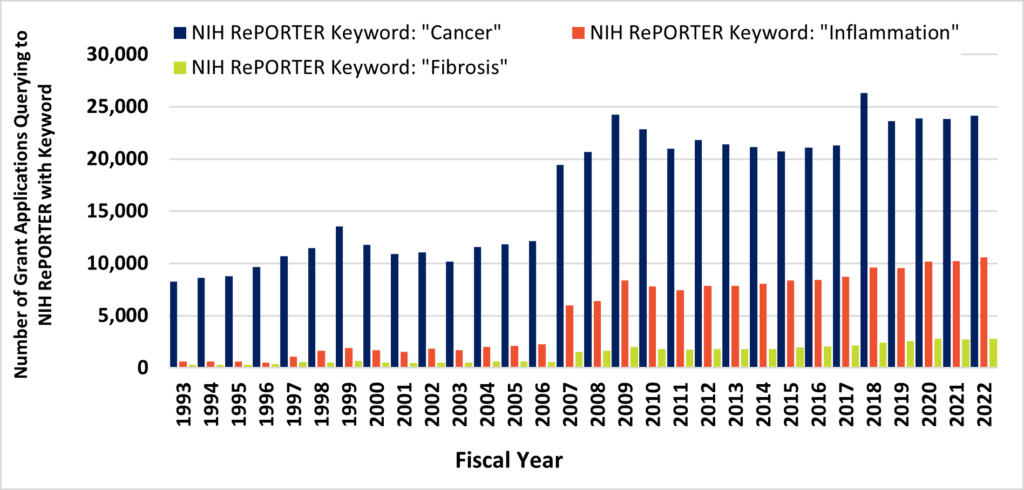
Above, Figure 1. Despite contributing to 45% of all deaths, fibrosis remains an unmet and neglected medical need. Figure 1A, the fraction of annual PubMed hits that queried to terms “cancer” (blue bars) and “inflammation” (orange bars) increased across three decades, while remaining stable for the query term, “fibrosis” (green bars). Figure 1B, the number of NIH grant awards that query to the terms “cancer” (blue bars) and “inflammation” (orange bars) increased across three decades, while overshadowing the number of grant awards that query to the term “fibrosis” (green bars).
Approximately 8% of all PubMed publications query to the term “fibrosis,” and this estimate has remained flat for three decades. Despite this, long-term trends demonstrate remarkable increases in journal activity for similar queries to PubMed via “cancer” or “inflammation” terms (Figure 1A). These are, of course, alternate disease classes that are presently targeted by a diverse armamentarium of experimental and marketed drugs. Mirroring annual cancer and inflammation-related publications are commensurate increases in the number of NIH-funded projects (Figure 1B). At long last, intensive research would seem to be starting to turn the tide against cancer after many years (Figure 2). According to the United States CDC, the annual percent of cancer’s mortality decreased from a high of 23% of all 1999 deaths (200.8 per 100K persons), down to below 20% by 2020 (144.1 per 100K). Nevertheless, queries to the NIH RePORTER web portal would suggest that the number of fibrosis-related funded projects reaches a level of only about 10% of cancer grant activity. The COVID-19 pandemic and lingering “long COVID” has particularly heightened recent interest in lung fibrosis, [5] which may continue to affect as many as 44.9% of those who endured serious infections. [6] Therefore, fibrosis remains a serious unmet medical need. [7]
Figure 2
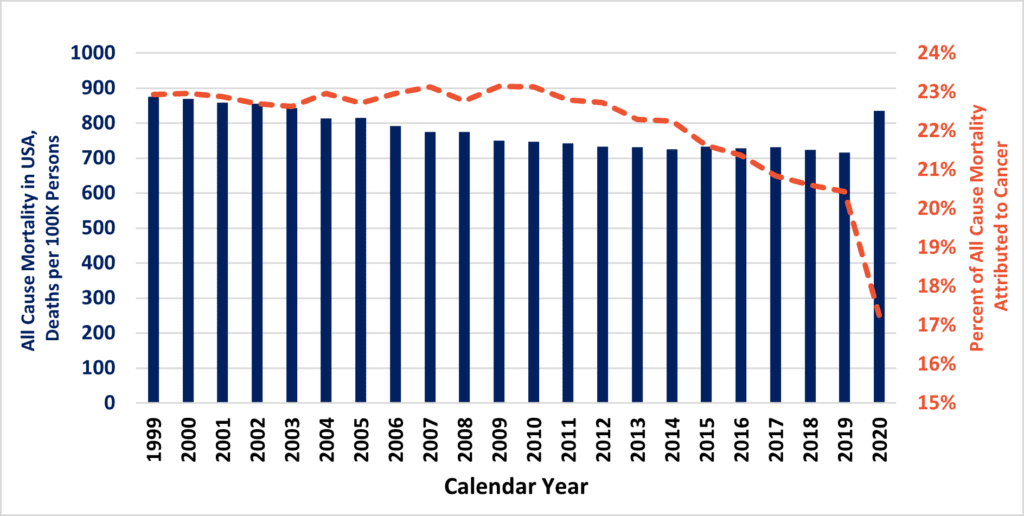
Above, Prior to 2020, USA all-cause mortality (blue bars) was slowly inching down. Of this, the fraction of deaths due to cancer (orange hatched line) also experienced a decline in the 2010s.
We Know Enough to Learn Much More
Both cancer and fibrosis share a resemblance to a wound-healing process gone awry. Sometimes, cancer and fibrosis co-conspire for tumor stromal barriers, pathological angiogenesis, and immune privilege in the same anatomical “crime scenes.” [8, 9] However, malignancy distinctly tends toward cell growth and genomic instability, with natural selection of variant clones for metastasis beyond the unhealed “wound.” In contrast, pathologic fibrosis stays put. That is, a fibrotic disease is the non-homeostatic release of excess, stiff extracellular matrix that precludes the normal restoration of a damaged and/or infected tissue.
The difference between the tendency for quick and regenerative wound healing in young children versus older adults might be evolutionarily hard-wired. [3] In the wild, deep wounds are sites of infection that could be cause for almost instant death from sepsis. With more energy/caloric resources invested into care of “tenderfoots” as future generations of mammals, the ancient tradeoff may alternately tip in favor of “quick and just good enough” scar formation as adults attain breeding and combat age. Here, the older siblings and parents could somewhat benefit from a tough scar’s prevention of excess blood loss and barrier function against invading pathogens, or repeated insults to the old injury site. (Just ask any guitarist, fiddler, or bassist; rough, calloused skin on fingertips isn’t so bad!)
The blurred ontology between infection response, inflammation, and fibrosis is supported by a massive literature on the interplay of immune cell types (neutrophils, macrophages, B cells, T cells), epithelial cells, and mesenchymal cells like fibroblasts. [7, 10, 11] Natural wound healing involves a choreography of these players’ entrances and exits from the lesion, along with characteristic changes in cytokine and chemokine milieu. In the young adult, scarring may gradually resolve over time as the tissue remodels. But in the elderly, this fibrosis might be closely tied to aging itself, perhaps due to “epigenetic noise” where cells gradually lose their identity and/or responsiveness to neighboring cues at the level of randomly silenced chromatin. [12, 13] The enduring presence of damage-signaling molecular patterns in a fibrotic lesion—populated by post-replicative, senescent cells addled by epigenetic nose—may elicit a low-level autoimmunity and/or what has been popularly called “inflammaging.” [11, 14, 15] Such local inflammation may in fact feed back into the pathology, with some T cell subsets (e.g., Th17) self-perpetuating this fibrotic lesion [10, 16] together with fibroblasts [17] in a specific context that can be heavily influenced by TGF-b/Smad signaling, along with IL-4, IL-6, IL-13 and other mediators. [3]
TGF-b recruits fibroblasts that, in turn, terminally differentiate into myofibroblasts, which are contractile cells of intermediate morphology between smooth muscle cells and fibroblasts. [18, 19] With lesions healing nominally, these die by apoptosis while the wound is resolved—gradually replaced by the familiar residents of soft tissue architecture. Yet in hypertrophic fibrosis, myofibroblasts may continue to perpetuate a TGF-b autocrine loop that suppresses proximal epithelial cell growth and induces endothelial- and epithelial to mesenchymal (EMT) transitions, [20] increasing the margins of the scarring. Obviously, fibrotic disease is more complex than briefly summarized here and context-dependent—varying between tissues and early to late phases of disease. [21] Along with highly context-dependent fibrosis presentation, there comes an inevitable challenge to treat the condition due to narrow windows of efficacy via molecular targeted drugs. [4, 9, 21] This includes pharmacopoeia to antagonize TGFb, where total systemic blockade is not without major toxicity. [9, 21]
MSCs & Their EVs/Exosomes – Thinking Globally, Acting Locally
Injected mesenchymal stromal/stem cells (MSCs) reportedly home toward in vivo toward injuries under certain experimental conditions, and they also may do so endogenously. [22, 23, 24] When implanted locally at the injury site—or artificially encapsulated in proximity—their secreted factors and vesicles are widely observed to contribute to rapid tissue regeneration in animal studies. [25, 26, 27] Consistent with MSCs’ original role as pericytes that activate near punctured blood vessels and support their repair, [27, 28, 29] MSCs are the basis for over 1500 worldwide clinical trials posted to international databases since 2011. [30] Their overall track record in human patients has been quite safe, and hence they comprise the active cellular material for approximately 10 approved therapies beyond the United States. [31] MSCs’ capability for artificial targeting, in situ, via topical paracrine secretome activity may open new avenues for combatting fibrosis that might otherwise be too risky for systemic small molecules or mAbs.
Potent anti-inflammatory, pro-angiogenic, anti-microbial, and anti-fibrotic features of MSCs are widely reported. However, MSCs also are a heterogeneous mix—sourced from different tissues via different isolation methods, from different donors who each exhibit different ages and health states. Today, the mechanisms of action of MSCs are strongly believed to be related to their secretory profiles. Still, the reductionist quest to isolate their most essential factors remains perplexingly elusive. [32] A confounding feature is that MSCs may also, themselves, differentiate into myofibroblasts and/or perpetuate fibrosis, being responsive to TGF-b. [33] Therefore, as a double-edged sword, it becomes imperative to fastidiously characterize MSCs according to ISCT guidelines [34, 35] and transparently publish all details of their sourcing, isolation, expansion, dosing, and administration methodology so that the state-of-the-art can advance along well-defined quality attributes (CQAs). [36]
Multiple differentiation states of MSCs may seem at first glance as an obstacle to future clinical translation. A steadily increasing volume of published research suggests this might be surmounted, however (Figure 3). MSCs are readily engineerable cell systems, so their broad profiles of regenerative “features” are not “bugs” at all. Our own group at RoosterBio—and many others—have demonstrated that the therapeutic phenotype of MSCs can be dramatically altered through priming pretreatments, [37, 38] high-performance media, [39] and genetic modification. [40] Among other means, heterologous overexpression of the putative anti-fibrotic hepatocyte growth factor (HGF) in MSCs might be particularly effective. [41] In addition, MSCs are prolific producers of extracellular vesicles (MSC-EVs), including exosomes. [42] cGMP-bioprocessed exosomes can be collected from “domesticated” MSC producer cell supernatants [43, 44, 45] that are analogous to the “workhorse” CHO cells for mAbs or HEK-293Ts that yield clinical lentivectors. These lipid-bound particles are themselves able to ameliorate ex vivo and in vivo disease models of fibrosis. [46] Likewise, MSC-EVs are customizable by genetic modification of the host cell and/or by artificial modifications that are post-harvest. [47] The surfaces of MSC-EVs could express multivalent, tissue-selective binding and internalization moieties (including scFvs), [48, 49] and their contents can be enriched with small molecule, protein, miRNA, siRNA, mRNA, or (epi)genome editor cargoes to radically alter the course of fibrotic or other modeled diseases. [50] Thus—by steering away from off-tissue effects as a designer drug delivery platform—both MSCs and MSC-EVs can attack multiple and simultaneous fibrotic molecular targets once dismissed as “undruggable.” [51]
Figure 3
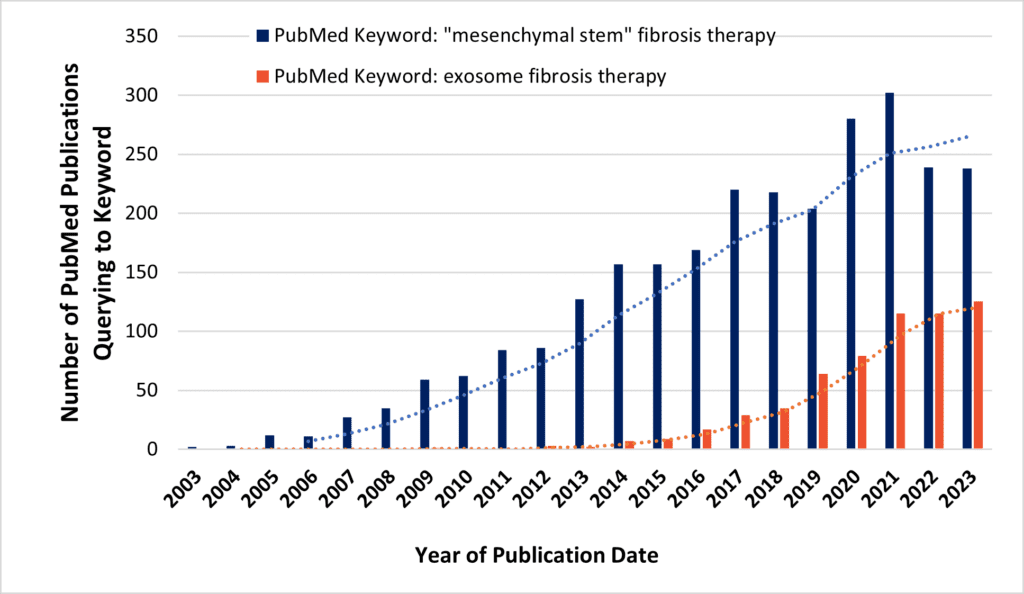
Above, PubMed search queries may indicate increasing research interest in use of MSCs (blue bars) or MSC-EVs/exosomes (orange bars) for novel anti-fibrotic therapies.
“Think globally, act locally,” exclaims many a bumper sticker. Like most tailpipe memes, it sounds nice enough—but this bromide is obviously difficult to pin down for practical specifics. Yet, one would be stumped to think of a more geographically dispersed cause of historical human misery than organ fibrosis. And one would be hard-pressed to imagine anything more local than a therapy within the human body, acting as a “magic bullet” with molecular and spatiotemporal specificity against a disease process. Too good to be true? Maybe not! With MSCs and their EVs, have we finally approached the ultimate example of a cause worth fighting for?
References
- Wynn TA. Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases. J Clin Invest. 2007;117(3):524-9; doi:10.1172/JCI31487
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199-210; doi:10.1002/path.2277
- Thannickal VJ, Zhou Y, Gaggar A, Duncan SR. Fibrosis: ultimate and proximate causes. J Clin Invest. 2014;124(11):4673-7; doi:10.1172/JCI74368
- Li X, Zhu L, Wang B, Yuan M, Zhu R. Drugs and Targets in Fibrosis. Front Pharmacol. 2017;8:855; doi:10.3389/fphar.2017.00855
- Verter F. A Role for MSC to Treat Coronavirus Patients. Part 2: Fibrosis in Survivors. https://celltrials.org/news/role-msc-treat-coronavirus-patients-part-2-fibrosis-survivors (2020). Accessed
- Hama Amin BJ, Kakamad FH, Ahmed GS, Ahmed SF, Abdulla BA, Mohammed SH, et al. Post COVID-19 pulmonary fibrosis; a meta-analysis study. Ann Med Surg (Lond). 2022;77:103590; doi:10.1016/j.amsu.2022.103590
- Nanchahal J, Hinz B. Strategies to overcome the hurdles to treat fibrosis, a major unmet clinical need. Proc Natl Acad Sci U S A. 2016;113(27):7291-3; doi:10.1073/pnas.1607896113
- Otranto M, Sarrazy V, Bonte F, Hinz B, Gabbiani G, Desmouliere A. The role of the myofibroblast in tumor stroma remodeling. Cell Adh Migr. 2012;6(3):203-19; doi:10.4161/cam.20377
- Akhurst RJ. Targeting TGF-beta Signaling for Therapeutic Gain. Cold Spring Harb Perspect Biol. 2017;9(10); doi:10.1101/cshperspect.a022301
- Zhang M, Zhang S. T Cells in Fibrosis and Fibrotic Diseases. Front Immunol. 2020;11:1142; doi:10.3389/fimmu.2020.01142
- Serrano-Lopez R, Morandini AC. Fibroblasts at the curtain call: from ensemble to principal dancers in immunometabolism and inflammaging. J Appl Oral Sci. 2023;31:e20230050; doi:10.1590/1678-7757-2023-0050
- Sinclair DA, Mills K, Guarente L. Accelerated aging and nucleolar fragmentation in yeast sgs1 mutants. Science. 1997;277(5330):1313-6; doi:10.1126/science.277.5330.1313
- Lu Y, Brommer B, Tian X, Krishnan A, Meer M, Wang C, et al. Reprogramming to recover youthful epigenetic information and restore vision. Nature. 2020;588(7836):124-9; doi:10.1038/s41586-020-2975-4
- Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244-54; doi:10.1111/j.1749-6632.2000.tb06651.x
- Moiseeva V, Cisneros A, Sica V, Deryagin O, Lai Y, Jung S, et al. Senescence atlas reveals an aged-like inflamed niche that blunts muscle regeneration. Nature. 2023;613(7942):169-78; doi:10.1038/s41586-022-05535-x
- Schmitt V, Rink L, Uciechowski P. The Th17/Treg balance is disturbed during aging. Exp Gerontol. 2013;48(12):1379-86; doi:10.1016/j.exger.2013.09.003
- Selman M, Pardo A. Fibroageing: An ageing pathological feature driven by dysregulated extracellular matrix-cell mechanobiology. Ageing Res Rev. 2021;70:101393; doi:10.1016/j.arr.2021.101393
- Majno G, Gabbiani G, Hirschel BJ, Ryan GB, Statkov PR. Contraction of granulation tissue in vitro: similarity to smooth muscle. Science. 1971;173(3996):548-50; doi:10.1126/science.173.3996.548
- Tai Y, Woods EL, Dally J, Kong D, Steadman R, Moseley R, et al. Myofibroblasts: Function, Formation, and Scope of Molecular Therapies for Skin Fibrosis. Biomolecules. 2021;11(8); doi:10.3390/biom11081095
- Marconi GD, Fonticoli L, Rajan TS, Pierdomenico SD, Trubiani O, Pizzicannella J, et al. Epithelial-Mesenchymal Transition (EMT): The Type-2 EMT in Wound Healing, Tissue Regeneration and Organ Fibrosis. Cells. 2021;10(7); doi:10.3390/cells10071587
- Pohlers D, Brenmoehl J, Loffler I, Muller CK, Leipner C, Schultze-Mosgau S, et al. TGF-beta and fibrosis in different organs – molecular pathway imprints. Biochim Biophys Acta. 2009;1792(8):746-56; doi:10.1016/j.bbadis.2009.06.004
- Zhao W, Phinney DG, Bonnet D, Dominici M, Krampera M. Mesenchymal stem cell biodistribution, migration, and homing in vivo. Stem Cells Int. 2014;2014:292109; doi:10.1155/2014/292109
- Szydlak R. Mesenchymal stem cells’ homing and cardiac tissue repair. Acta Biochim Pol. 2019;66(4):483-9; doi:10.18388/abp.2019_2890
- Jin W, Liang X, Brooks A, Futrega K, Liu X, Doran MR, et al. Modelling of the SDF-1/CXCR4 regulated in vivo homing of therapeutic mesenchymal stem/stromal cells in mice. PeerJ. 2018;6:e6072; doi:10.7717/peerj.6072
- Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9(1):11-5; doi:10.1016/j.stem.2011.06.008
- Pittenger MF, Discher DE, Peault BM, Phinney DG, Hare JM, Caplan AI. Mesenchymal stem cell perspective: cell biology to clinical progress. NPJ Regen Med. 2019;4:22; doi:10.1038/s41536-019-0083-6
- Caplan AI. MSCs: The Sentinel and Safe-Guards of Injury. J Cell Physiol. 2016;231(7):1413-6; doi:10.1002/jcp.25255
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3(3):301-13; doi:10.1016/j.stem.2008.07.003
- Slukvin, II, Kumar A. The mesenchymoangioblast, mesodermal precursor for mesenchymal and endothelial cells. Cell Mol Life Sci. 2018;75(19):3507-20; doi:10.1007/s00018-018-2871-3
- celltrials.org. available from Cell Trials Data. https://celltrials.org/public-cells-data/msc-trials-2011-2020/ (2022). Accessed
- Hildreth C. MSC Therapies: Globally Approved Mesenchymal Stem Cell Therapeutics. https://bioinformant.com/msc-therapies/ . (2019). Accessed
- Harrell CR, Fellabaum C, Jovicic N, Djonov V, Arsenijevic N, Volarevic V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells. 2019;8(5); doi:10.3390/cells8050467
- Qin L, Liu N, Bao CL, Yang DZ, Ma GX, Yi WH, et al. Mesenchymal stem cells in fibrotic diseases-the two sides of the same coin. Acta Pharmacol Sin. 2023;44(2):268-87; doi:10.1038/s41401-022-00952-0
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315-7; doi:10.1080/14653240600855905
- Krampera M, Galipeau J, Shi Y, Tarte K, Sensebe L, Therapy MSCCotISfC. Immunological characterization of multipotent mesenchymal stromal cells–The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15(9):1054-61; doi:10.1016/j.jcyt.2013.02.010
- Carmen J, Burger SR, McCaman M, Rowley JA. Developing assays to address identity, potency, purity and safety: cell characterization in cell therapy process development. Regen Med. 2012;7(1):85-100; doi:10.2217/rme.11.105
- Kratchmarova I, Blagoev B, Haack-Sorensen M, Kassem M, Mann M. Mechanism of divergent growth factor effects in mesenchymal stem cell differentiation. Science. 2005;308(5727):1472-7; doi:10.1126/science.1107627
- Boregowda SV, Krishnappa V, Haga CL, Ortiz LA, Phinney DG. A Clinical Indications Prediction Scale Based on TWIST1 for Human Mesenchymal Stem Cells. EBioMedicine. 2016;4:62-73; doi:10.1016/j.ebiom.2015.12.020
- Snyder J, Carson, J. The Story Behind the Media. https://www.roosterbio.com/blog/the-story-behind-the-media/ (2022). Accessed
- Gonzalez-Junca A, Liu FD, Nagaraja AS, Mullenix A, Lee CT, Gordley RM, et al. SENTI-101, a Preparation of Mesenchymal Stromal Cells Engineered to Express IL12 and IL21, Induces Localized and Durable Antitumor Immunity in Preclinical Models of Peritoneal Solid Tumors. Mol Cancer Ther. 2021;20(9):1508-20; doi:10.1158/1535-7163.MCT-21-0030
- Meng HF, Jin J, Wang H, Wang LS, Wu CT. Recent advances in the therapeutic efficacy of hepatocyte growth factor gene-modified mesenchymal stem cells in multiple disease settings. J Cell Mol Med. 2022;26(18):4745-55; doi:10.1111/jcmm.17497
- Yeo RW, Lai RC, Zhang B, Tan SS, Yin Y, Teh BJ, et al. Mesenchymal stem cell: an efficient mass producer of exosomes for drug delivery. Adv Drug Deliv Rev. 2013;65(3):336-41; doi:10.1016/j.addr.2012.07.001
- Lenzini S. Extracellular Vesicle/Exosome Upstream Process Development: Maximizing Productivity to Accelerate Clinical Adoptio. https://www.roosterbio.com/blog/extracellular-vesicle-exosome-upstream-process-development-maximizing-productivity-to-accelerate-clinical-adoption/ (2022). Accessed
- Jung J, Lenzini, S. Extracellular Vesicle/Exosome Downstream Process Development Part I: Leveraging Filtration Technologies for Scalable EV Preparation. https://www.roosterbio.com/blog/extracellular-vesicle-exosome-downstream-process-development-part-i-leveraging-filtration-technologies-for-scalable-ev-preparation/ (2022). Accessed
- RoosterBio. From Elephant Ears to Ambr® Waves: A New Path Toward Scale & Abundance for Biomanufactured hMSCs & Their Exosomes. https://www.roosterbio.com/blog/from-elephant-ears-to-ambr-waves-a-new-path-toward-scale-abundance-for-biomanufactured-hmscs-their-exosomes/ (2022). Accessed
- Mansouri N, Willis GR, Fernandez-Gonzalez A, Reis M, Nassiri S, Mitsialis SA, et al. Mesenchymal stromal cell exosomes prevent and revert experimental pulmonary fibrosis through modulation of monocyte phenotypes. JCI Insight. 2019;4(21); doi:10.1172/jci.insight.128060
- Kojima R, Bojar D, Rizzi G, Hamri GC, El-Baba MD, Saxena P, et al. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat Commun. 2018;9(1):1305; doi:10.1038/s41467-018-03733-8
- Wang JH, Forterre AV, Zhao J, Frimannsson DO, Delcayre A, Antes TJ, et al. Anti-HER2 scFv-Directed Extracellular Vesicle-Mediated mRNA-Based Gene Delivery Inhibits Growth of HER2-Positive Human Breast Tumor Xenografts by Prodrug Activation. Mol Cancer Ther. 2018;17(5):1133-42; doi:10.1158/1535-7163.MCT-17-0827
- Stranford DM, Simons LM, Berman KE, Cheng L, Lucks JB, Hultquist JF, et al. Bioengineering multifunctional extracellular vesicles for targeted delivery of biologics to T cells. bioRxiv. 2022:2022.05. 14.491879;
- Wan T, Zhong J, Pan Q, Zhou T, Ping Y, Liu X. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci Adv. 2022;8(37):eabp9435; doi:10.1126/sciadv.abp9435
- Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478); doi:10.1126/science.aau6977
- Image. “Human fibrotic disease in the style of Rembrandt” prompt, Craiyon AI, 2023, https://www.craiyon.com/.
