Exosomes / Extracellular Vesicles (EVs) are an emerging frontier of controllable drug delivery technology. With apologies to the game series creators, the nascent extracellular vesicle therapeutic landscape is the exemplar of a “Choose Your Own Adventure.” Like many adventures, explorations into the EV wilds begin by choosing the right-fitting gear before trailblazing through impactful discoveries. Together, RoosterBio (Crystal Cruz, Bioprocessing Consultant) and VivaZome Therapeutics (David Haylock, Ph.D., CEO, VivaZome Therapeutics) recently described one such journey in a joint webinar titled “Manufacturing Advances Unlock the Therapeutic Potential of MSC-Derived Exosomes.” 1
In Part One of the webinar, Crystal Cruz summarized the wares and services offered to the novice “EVenturer” via RoosterBio—a seasoned outfitter for advanced therapeutics and regenerative medicine developers in business for more than 13 years. Part Two of the program featured Dr. Haylock’s exciting work with VivaZome, titled “Bone marrow-MSC EVs with anti-inflammatory activity and their use in treating retinal degeneration and traumatic brain injury.” This webinar concluded with a lively 10-minute session of Q&A with questions from the viewers directed to Cruz and Haylock.
Challenges & Solutions
When outfitting for an exosome / extracellular vesicle program, Crystal Cruz showed that early pivotal bioprocess decisions made in parallel with preclinical development will help the investigator pass through some known challenges with steady momentum and resiliency. As is well known, extracellular vesicles from human mesenchymal stromal/stem cells (hMSCs) are promising in several ways: they’re potentially targetable like monoclonal antibodies (mAbs), they can carry native and artificial cargoes like liposomes and/or LNPs, and they embody many of the same therapeutic bioactivities as their living MSC producer cells. However, the biggest challenge with MSC-EVs is whether one can manufacture a consistent product. That is, we need optimized upstream and downstream processes with outcomes characterized by the best analytical tools. Luckily, specialized EV process development experts like the RoosterBio team offer solid solutions that are seamlessly translating R&D successes into clinical breakthroughs. These include EV-specific bioprocesses with tailor-fit media for optimal cell expansion and extracellular vesicle collection, scalable bioreactor and DSP platforms such as those devised by Sartorius and Repligen, and the right CQA monitoring solutions like NanoFCM (Figure 1).
| Historical Challenges | Modern Solutions |
|
|
Figure 1. Obstacles for extracellular vesicle preclinical and clinical product developers can be scaled with the right solutions, available now.
Leverage Scalable Platforms for High Upstream Cell Productivity & Favorable Downstream Purity & Potency
The planning for one’s custom “Choose Your Own [exosome/extracellular vesicle] Adventure” story can benefit from experts who have contributed to this journey before. Crystal Cruz explained how critically important scalability is for future clinical products made from extracellular vesicles. Like most good plans, an extracellular vesicle process needs to be devised “in reverse” with the end goal at the forefront: a product with defined specifications, manufactured at scales realistic for all uses, for use in both human patients and beyond. A key point is that while extracellular vesicle doses could likely be concentrated and stockpiled more easily than live MSCs, they may yet require threefold more cells to produce than traditional cell therapies. Thus, RoosterBio has learned from experience that bioreactor culture may be more favorable than tandem 2D cell stacks to manufacture clinic-sized doses of extracellular vesicles. Although 2D methods are “hands-on” for many startup programs and certainly viable, RoosterBio is a rare solutions provider who can help the investigator navigate easy transitions into a “3D” process that could yield quadrillions of extracellular vesicles needed for Phase III trials and post-market approval.
Anyone in a 101-level chemistry wet lab class will tell you: each step erodes a final product’s percent yield, no matter how careful the student is. For complex extracellular vesicle biologic therapeutics, there’s also an inevitable tradeoff between input raw materials’ bulk quantity and the fine quality that is output from downstream isolation steps. And yes, with human patients, purity of the final product also matters for a passing grade! These drug products demand a defined purity to generate dose lots with consistent bioactivity, potency, and a safe window of efficacy. Cruz outlined how RoosterBio’s practical EV experience in process steps and instrument platforms can navigate the investigator client within the desired standard of EV purity—whether the goal is quick R&D data or clinical doses. With this domain expertise in downstream processing (DSP), it is thus possible to right-scale programs that allocate the correct volumes and formats of cellular and media raw materials…and avoid costly mistakes (Figure 2).
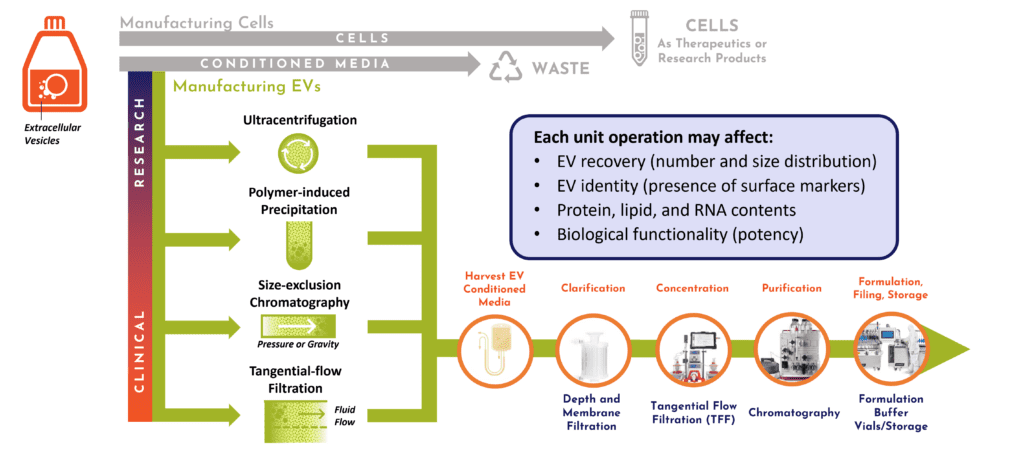
Figure 2. DSP decisions are dependent on output and scale. Upstream, an extracellular vesicle bioprocess for preclinical or clinical studies is affected by the secretory profile of producer cells into harvested conditioned media. Downstream, it is driven by the systems employed for clarification, concentration, purification, formulation, filling, and storage.10
Analytics: Seeing is Believing
Minimal Information for Studies of Extracellular Vesicles (MISEV) guidelines have been published for a decade 2, 3 and are now completing the edits for a major revision, due in 2023. 4 These aim to improve the quality of peer-reviewed extracellular vesicle / exosome science such that the state-of-the-art can advance. Cruz emphasized that critical quality attributes (CQAs) to define extracellular vesicle products ought to be well-informed 5 by the MISEV community’s recommendations for best practices. Such CQAs may include basic features like number and size distribution of particles, proportion of particles that are lipid-bound, maintenance of putative surface markers, proportion and type of nucleic acid luminal cargoes, and potency. Nevertheless, each new EV product is likely to be different—and hence also definable by its unique bioactivity for the specific clinical indication. Because of this, a development partner such as RoosterBio may help fast-track bewildered clients en route to the clinic with custom cell engineering and assay development of robust test systems that are directly tailored to the product specs (Figure 3).
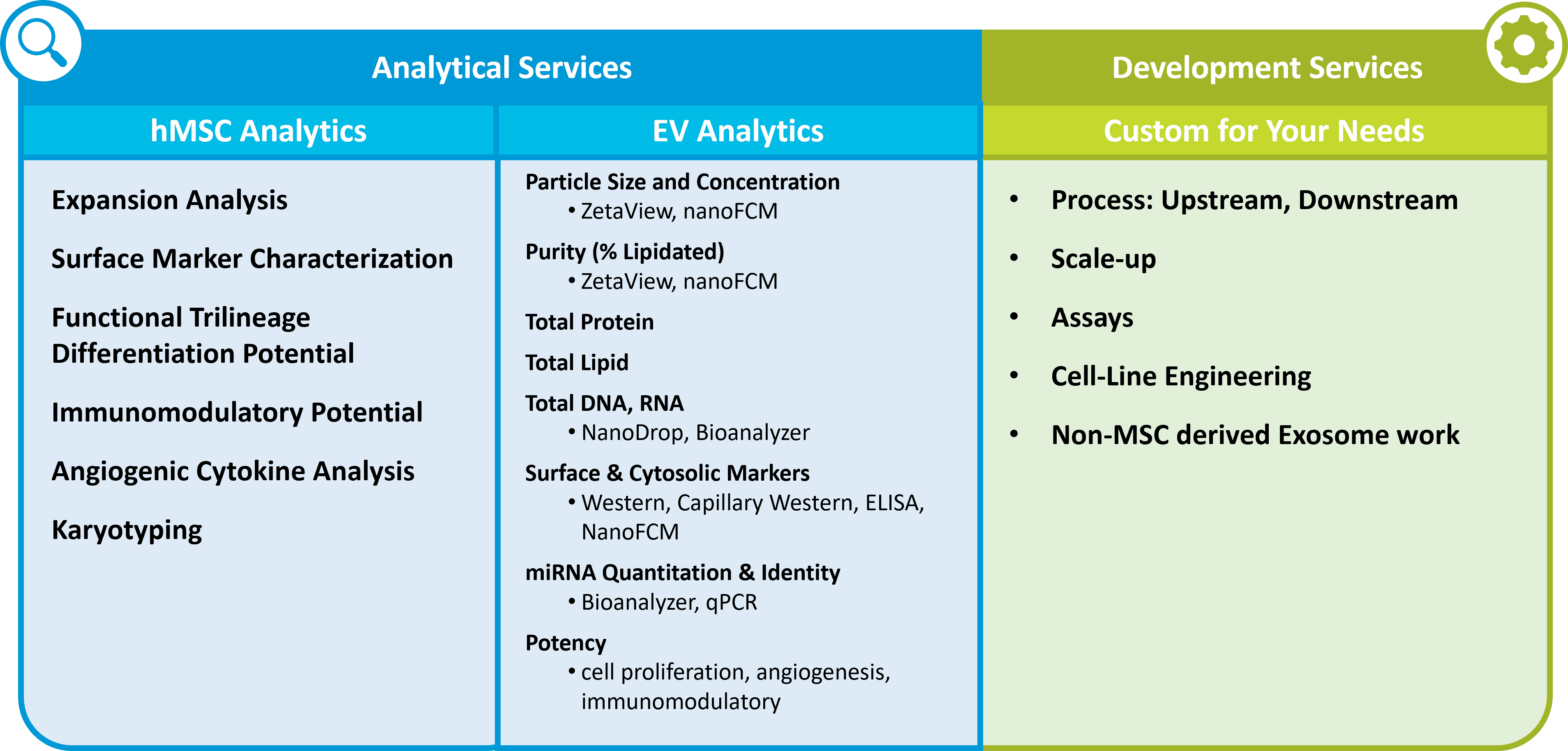
Figure 3. Examples of important off-the-shelf and custom analytical and assay development services offered by competent MSC-EV solutions providers such as RoosterBio.
VivaZome Therapeutics Leads the Way into a New Therapeutic Ecosystem
It was a marvelous privilege to welcome Dr. David Haylock, Ph.D., CEO, for his reporting of recent findings of relevance to ophthalmic diseases. At the 16:28 minute mark, Crystal Cruz turned over the webinar to Dr. Haylock where he described VivaSome’s work in collaboration with Melbourne’s LaTrobe University, Australian National University, University of Queensland, CSIRO, and others. VivaZome also employed RoosterBio cells and media toward this work. In this ~30-minute talk, Haylock summarized compelling data that employed MSC-EVs on both cell culture screening and in vivo models of acute retinal damage.
VivaZome aims to harness the potential of its proprietary exosome and cell engineering platform technologies. Combining technology with bioprocess expertise, VivaZome focuses on retinal degeneration, traumatic brain injury, and other neurological conditions as avenues for possible clinical translation. “Exosomes have some really amazing properties 6 that make them suitable for therapeutics,” he said. “They’re nano-sized vesicles released naturally by most cells in the body” and important and potent effectors of cell-cell communication, perhaps responsible for the activity of cell therapies via their unique mix of molecular cargo. They can exhibit activities similar to that of their parental MSCs, such as angiogenic, anti-fibrotic, anti-inflammatory, and regenerative effects. Notably, Haylock reports that exosomes can cross the blood-brain barrier, making them excellent vehicles to ameliorate neurodegenerative conditions.
Extracellular Vesicle Manufacturing is Agnostic Regarding Cell Type & Upstream & Downstream Process (20:38)
At VivaZome, Dr. Haylock and team combine modular sub-process segments into a durable yet flexible process. The “upstream” inputs are human adherent cells with decent exosome productivity—preferentially having a proven clinical track record (e.g., cGMP manufacturable hMSCs) and expandable with validated, closed-system, cGMP-compliant bioreactor technology. The cell culture supernatant serves as starting substrate for the downstream steps: separation & concentration and next, the formulation. In experiments depicted thereafter, hMSC cell culture supernatants captured in RoosterCollect™ from two of RoosterBio’s bone marrow donors were treated to successive steps of centrifugation, tangential flow filtration (TFF), size exclusion chromatography (SEC), and an additional 4000g concentration spin with an Amicon 10kD cutoff. The final preparation of EVs was a highly concentrated 5 x 1011 particles per mL. These two MSC cell sources (“-305” and “-310”) were chosen due to their well-characterized phenotypic profiles provided by RoosterBio that implied stronger anti-inflammatory properties.
In Vitro Validation of hMSC-EVs’ Anti-Inflammatory Effects (29:30)
To screen for possible anti-inflammatory effects via the two hMSC-EV sources, VivaZome worked with Melbourne’s Crux Biolabs’ cell-based, 7-plex assay system that used a model of LPS-stimulated human PBMCs and cytokine level readouts. In the presence of varying concentrations of MSC-EVs, there was observed apparent suppression of IL8 and/or IL6 release by the PBMCs.
This initial work inspired confidence to proceed with more complex in vivo studies via model systems devised by Clear Vision Research in concert with Australian National University. “They, like us, have a vision to exploit small EVs as a therapeutic modality for treating retinal degeneration,” emphasized Dr. Haylock. Clear Vision’s recent 2020 publication, “Small-Medium Extracellular Vesicles and Their miRNA Cargo in Retinal Health and Degeneration: Mediators of Homeostasis, and Vehicles for Targeted Gene Therapy” (DOI: 10.3389/fncel.2020.00160) 7 was groundbreaking in its demonstration of how exosome miRNA cargoes may be ablated in lesions of retinal degeneration, thereby driving disease progression.
Amelioration of Retinal Degeneration In Vivo (31:35)
Together with Clear Vision Research, VivaZome tested 1ul doses of 2.0 x 109 MSC-EVs (-/+) via intravitreal injection into mice subjected to intense, eye-damaging bright light. 8 The effects of these treatments on retinal function, retinal morphology, apoptotic cell death, microglial/macrophage presence in tissue arrays, and gene expression signatures of oxidative stress were then monitored.
Results? Electroretinogram (ERG) tests showed significant protection via the extracellular vesicles from both MSC donors. Also, TUNEL stains for apoptotic (dead) cells in the total retina and superior retinal were also decreased via EV treatment, particularly with the “-305” hMSC donor source. Looking at presence of ameboid (activated) microglial/macrophages by IBA1+ staining, it appeared that the “-305” sourced MSC-EVs also exhibited a stronger protective effect on this histological readout than the “-310” donor. From these results, Dr. Haylock concluded that bone marrow-derived MSCs are a suitable candidate cell type for exosome product manufacture.
Customized Exosomes (37:05)
Dr. Haylock then briefly shifted gears of the talk to emphasize another shiny angle of exosome technology— namely, that there can be advantages to further “customize” these particles. He listed several options to drive the technology forward. These include enhancement of tissue and cellular targeting, improved manufacture and stability, addition or deletion of lumen-surrounded cargo, enhancement of biofunction on target cells/tissues, theragnostic tracking, and opportunities for novel and useful IP generation.
Using supernatants collected from a different cell type (HEK293), VivaZome showed how exosomes can be selectively retargeted using a glycosylation-modified Lamp2b fused with a brain-trophic RVG peptide. This effort was exhibited in a poster abstract at ANSEV-2022, titled “Bioengineering exosomes to enhance brain targeting in a mouse traumatic brain injury model.” 9 Exosomes engineered in this or similar ways might one day comprise a delivery system for therapeutic cargoes that protect the brain from traumatic brain injuries and/or neurodegenerative conditions.
Questions & Answers (43:00)
Several questions were raised in the webinar’s last ten minutes, diligently answered by both VivaZome’s Dr. David Haylock and RoosterBio’s Crystal Cruz. These included:
- What concentrations of extracellular vesicles were used in the in vitro assays?
- Is it preferable to show the extracellular vesicle concentration as particle number of by protein content?
- When or if high concentrations of extracellular vesicles are used, is there a saturating upper limit on biological effects observed?
- Are the reported “cell lines” of hMSCs (-305 and -310) established cell lines or primary cells?
- Is there evidence of immune rejection of human MSCs when injected into mice?
- How long can the MSCs from RoosterBio be maintained in vitro?
- With extracellular vesicle therapeutics being such a young field to explore—and only now entering trials—what are some thoughts on their outlook for clinical progress and possible obstacles along the way?
- What other active residual excipients might be in the formulation of the EVs (other than EVs)?
- Can you talk about various ways to engineer extracellular vesicle cargoes or displayed targeting handles?
- When RoosterBio talks with customers, are there common cargo loading modalities that are commonly asked about or used?
- Any closing thoughts?
What were the answers to these questions? At the risk of dangling carrot before this blog’s readers, you’ll definitely need to watch the webinar for the best exposure to the key details!
In conclusion, RoosterBio is extremely pleased with the strong audience turnout and response to this informative seminar—and of course—for the generous contribution of Dr. Haylock’s time across an ocean and 14 time zones. Please keep in touch with us, as this is an “adventure” that tells a continuing story.
References
- Cruz, C., Haylock, D. Manufacturing Advances Unlock the Therapeutic Potential of MSC-Derived Exosomes. https://tinyurl.com/roosterwebinar202306.
- Lotvall, J.; Hill, A. F.; Hochberg, F.; Buzas, E. I.; Di Vizio, D.; Gardiner, C.; Gho, Y. S.; Kurochkin, I. V.; Mathivanan, S.; Quesenberry, P.; Sahoo, S.; Tahara, H.; Wauben, M. H.; Witwer, K. W.; Thery, C., Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 2014, 3, 26913. 3402/jev.v3.26913
- Thery, C.; Witwer, K. W., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018, 7 (1), 1535750. 1080/20013078.2018.1535750
- Revision Committee. Minimal information for studies of extracellular vesicles 2022 (MISEV2022): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2018 guidelines. https://isev.memberclicks.net/assets/docs/MISEV22%20Draft.pdf.
- Cruz, C. From Benchmark to Airborne: MISEV Guidelines Inspire Fledgling EV/Exosome Analytics Services. https://www.roosterbio.com/blog/from-benchmark-to-airborne-misev-guidelines-inspire-fledgling-ev-exosome-analytics-services/.
- Claridge, B.; Lozano, J.; Poh, Q. H.; Greening, D. W., Development of Extracellular Vesicle Therapeutics: Challenges, Considerations, and Opportunities. Front Cell Dev Biol 2021, 9, 734720. 3389/fcell.2021.734720
- Wooff, Y.; Cioanca, A. V.; Chu-Tan, J. A.; Aggio-Bruce, R.; Schumann, U.; Natoli, R., Small-Medium Extracellular Vesicles and Their miRNA Cargo in Retinal Health and Degeneration: Mediators of Homeostasis, and Vehicles for Targeted Gene Therapy. Front Cell Neurosci 2020, 14, 160. 3389/fncel.2020.00160
- Wooff, Y.; Cioanca, A. V.; Wills, E.; Chu-Tan, J. A.; Sekar, R.; Natoli, R., Short exposure to photo-oxidative damage triggers molecular signals indicative of early retinal degeneration. Front Immunol 2023, 14, 1088654. 3389/fimmu.2023.1088654
- Chen, M., Krishna, S., Haylock, D., Reutens, D. Featured Abstract – Bioengineering exosomes to enhance brain targeting in a mouse traumatic brain injury model. Journal of Extracellular Vesicles 2023, 12 (S2), e12344. https://doi.org/10.1002/jev2.12344
- Smith, J. A.; Ng, K. S.; Mead, B. E.; Dopson, S.; Reeve, B.; Edwards, J.; Wood, M. J.; Carr, A. J.; Bure, K.; Karp, J. M., Extracellular Vesicles Commercial Potential As Byproducts of Cell Manufacturing for Research and Therapeutic Use. Extracellular Vesicles. BioProcess International 2015, 13 (4).

 EV-specific Bioprocess — RoosterBio Proprietary Medias
EV-specific Bioprocess — RoosterBio Proprietary Medias