“If I can make it there, I’ll make it anywhere” – Frank Sinatra
Leave it to Ol’ Blue Eyes to say it bold and proud. Once crooning to his beloved metropolis (NYC), he might as well have had low earth orbit (LEO) in mind. For fresh newcomers, both locales have offered costly and tight living arrangements, challenging commutes, and tough working conditions. Yet who can deny the spectacular vistas and interdisciplinary opportunities to change history in either spot? This brief blog will explore the challenges and promise of employing mesenchymal stem/stromal cells (MSCs) as cellular workhorses for astronauts, cosmonauts, and taikonauts for bioprinting applications in microgravity. Surely, if MSCs can generate valuable data and products in outer space, they can make them anywhere?
Why MSCs in Space?
Investigators eagerly turn to MSCs on Earth because they can be obtained in bulk, have normal karyotypes, differentiate into other useful cell types, and exhibit an extended but not indefinite replicative capacity. They have served as quality-controlled source material for at least 1,500 global human clinical trials on record and are believed to mediate various regenerative functions in mammalian physiology as “emergency first responders” to injury or infection. Compared with other primary cell types, MSCs are quite hardy, radioresistant to even 10 Gy of ionizing radiation, [1] and rarely fouled by culture contamination (perhaps due to their secreted antimicrobial [2] peptides). (So… “Won’t you please take them alone for a ride?”)
Space travelers beyond Earth’s Van Allen Belts face nontrivial risks of acute and/or chronic radiation sickness during passage to the Moon, Mars, and asteroids. [3] It’s reported that MSCs and/or their secretomes can mitigate progressive acute radiation damage in vivo and symptoms [4] related to chronic radiation syndrome. Also of benefit to astronauts—who may suffer from broken bones, head injuries, sudden loss of air pressure, puncture wounds, immune system collapse, sarcopenia, etc.—MSCs may promote general healing through wound-plugging, anti-inflammation, recruitment of macrophages, and pro-angiogenic signaling. [5] Naturally, it follows that future medical crews could have at liberty cocktails of gene-programmed medicines for secretion by the MSCs as skeletal muscle implanted “bioreactors.” [6]
One major benefit of MSCs is that they’re not cell lines. They weren’t expanded out of tumor isolates with unstable genomes and bizarre phenotypes. MSCs hence comprise an appropriate model system for cell and tissue culture to emulate natural cell behavior—OR what happens to “normal” human cells when placed in non-natural environments (e.g., the International Space Station; ISS). Therefore, MSCs may be practical subjects for travel into space because of the diverse ways they can be studied and because of their utility as raw cellular materials for high-value tissue products.
In a critical first step, MSCs blasted off on a ride to the ISS on February 19, 2017 for a study led by Professor Abba Zubair of Mayo Clinic Florida. [7] They were thawed preflight and loaded onto BioServe single-well BioCells, which are certified for space flight, and then placed in the BioServe SABL cell culture unit that maintains 5% CO2 and constant 37oC temperature. After 7- and 14-day timepoints the MSCs were cryopreserved at -95oC prior to their return home. By all measures they proliferated normally in microgravity, maintaining chromosomal integrity and the canonical ability to differentiate into adipose, bone, and cartilage. These simple experiments trailblazed a path for more complex cell engineering experiments and applications of MSCs beyond Earth, including bioprinting.
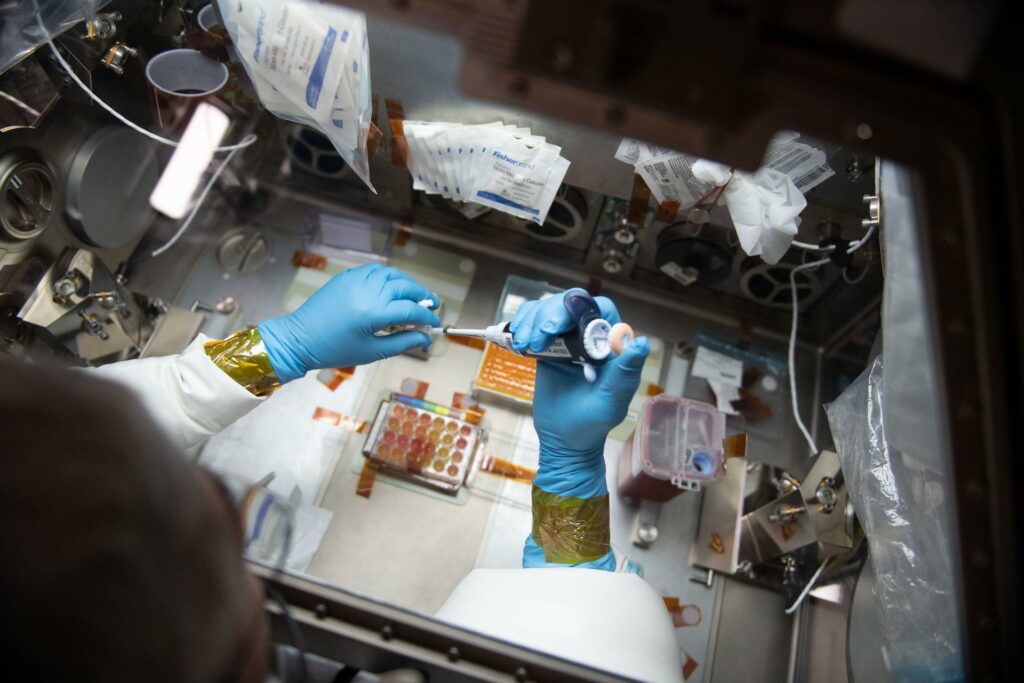
Astronaut Problems for MSCs to Solve
In the most basic sense, 3-dimensional bioprinting (bio-3DP) involves the concerted activity of three biocompatible components: a (1) scaffold or growth matrix, (2) a bioink, and (3), a printing device. The scaffold is the “home” for the cells that enable attachment and expansion. The bioink contains the live cells (or mix of cells) that deposit on or into the scaffold; it may be enriched in growth factors, matrix proteins, microcarriers, or have an innate gel-like viscosity. The printer is the device (e.g., extrusion, inkjet, melt electrowriting, or laser/lithography) that ejects, deposits, positions, or channels the bioinks within and/or onto the scaffold. Cell-enriched bioinks and scaffolds often blend in their properties such that the cells can be layered, ordered, or deposited according to a pre-programmed design CAD file. On the other hand, some scaffolds might also be printed partially or fully separated from the bioink. Scaffolds can be fashioned from hard or soft materials or be porous, channeled, or impermeable—precisely where desired at each three-dimensional coordinate locus. The combined printed tissue can also be dynamic while it matures during prep, controllable via photochemistry, acoustics, biodegradable matrices, and temperature-controllable phase changes. Obviously, there are multitudes of modalities for 3DP with biological materials in space or on the ground, as expertly reviewed by Van Ombergen, et. al (2023) [8] and by Sarabi, et. al (2023). [9]
Why would space travelers wish to play with 3DP capabilities on board? It’s not just a toy. As a kind of additive manufacturing (AM), 3DP would grant autonomy to rapidly improvise and prototype with reduced necessity for a spare parts inventory under the extremely limiting constraints on storage and raw materials. (It might spare astronauts a reprise of cobbling together a solution with duct tape, cardboard, and plastic bags.) It could enable them to burn through far fewer supplies and kilowatts on board the vessel or base, or possibly equip them to “live off the land” to utilize local resources. Alternatively, 3DP could allow the construction of an assembly of simple components for LEGO-like fabrication into a complex system. These same general principles can obviously be applied to an interplanetary med lab.
The first problem that bio-3DP solves is that it can be instrumental toward adaptation of cells to the microgravity, itself. On Earth’s surface, MSCs deposit to the bottom of a 2D flask where they can attach, spread, and resume population doublings after a split. However, cells cannot settle when in perpetual freefall around our globe at 17,500 miles per hour; if they cannot attach to a surface, many types die from anoikis. [10] Technical solutions will vary, but some might involve prior attachment within a pre-allotted, standardized bioink with digestible microcarriers before launch—or kept in a kind of suspended animation [11, 12] or cryostorage until 3DP seeding into the scaffold in situ. Larger scale use of MSCs without gravity thus involves cells cultured in a non-planar fashion, at much higher density, and with media that are highly enriched. It’s even conceivable to co-culture MSCs and engineered Chlorella vulgaris, [13] for example, where this microalgae-laden bioink could secrete recombinant survival factors to fortify and help condition the MSC for extended viability.
Another problem that bio-3DP might solve is the variable quality of engineered tissues, organoids, or organs. When fabricated in space, they would likely be greatly improved. Why? On the ground, cell settling can be an issue when attempting to make fully homogenized tissues or tissue layers. This earthbound challenge is somewhat circumvented by printing via highly viscous, soft bioinks. However, for adherent cells, these solutions are not necessarily optimal for cell viability or function and sometimes cause the artificial tissue to collapse in its own weight. In contrast, in space’s microgravity, it becomes possible to completely mix a dense solution of suspended cells in a low viscosity medium, where the ink is then filled into a chambered pre-printed scaffold with very equal distribution of cells—and no sedimentation. Such a degree of leverage over tissue engineering is unprecedented and is predicted to be a source of major breakthroughs for human health.
The earliest applications of bio-3DP in LEO may involve LOCs (Labs-on-Chips) that embody mini organoids or tissue models. Many of these will be seeded with MSCs with genetically programmable biosensing with embedded, promoter-controlled, logical computation. For long-duration missions in deep space, such cell systems could facilitate bespoke and personalized diagnostics for monitoring crew health. The austere off-world environment may well prove to be the ultimate “mother of invention” that incubates new earthbound technologies.
In addition to modeling and diagnosing disease, MSCs in bioinks could be useful for treating it. These cells demonstrate greatly improved persistence in vivo when implanted along a viable matrix, scaffold, or tissue. Accordingly, bio-3DP capabilities would enable MSCs to recapitulate their physiologic role with greater potency and efficacy for sick and injured astronauts. MSCs are also major support cells for organs as putative denizens of interstitial and perivascular tissues; as such, today’s complex ongoing R&D efforts to engineer organs rarely omit MSCs from the mix of other distinct ingredients like epithelial, endothelial, neuronal, or hematopoietic cell types. In 2049, if an ill-fated spacecraft was roasted by a powerful coronal mass ejection, cartridges of MSC bioinks might help formulate bespoke new thymuses (together with HSCs and TECs) for each crew member and recharge their depleted T cells. Or, heaven forbid, someone gets cancer 200,000,000 kilometers from home. In that case, don’t forget to pack the DNA printer; then, synthesize an integrated gene network to convert engineered MSCs into an artificial gland to secrete Ipilimumab or Trastuzumab along with synergistic adjuvants.
Another role where MSCs could enrich space morale (as well as supplement necessary protein) is in the creation of novel cuisines based on synthetic meat. Non-human (e.g., bovine, porcine, galline, ovine, piscine, caridean) MSCs might be cultured along with their respective myocytes and endothelial cells, plus artificial “spices” programmed into microalgal symbiont additives. It might well be less costly and less cumbersome to grow a meatball in space than to ship it in the cargo’s deep freeze unit? Bio-3DP is absolutely required to recapitulate the textures in such an austere environment, and the flavors might well be “out of this world…”
MSC Problems for Astronauts to Solve
Frivolity aside, it would be onerous to generate a credible business case for off-world bio-3DP unless millions of real-world customers would be willing to pay for such products. The microgravity of space would need to serve as an essential process parameter for the biomanufacturing of a superior life sciences commodity. It would require raw materials shipped from Earth’s surface to LEO, then transported back with total reliability, regulatory compliance, and safety. Yet even accounting for the full product life cycle of multiple inputs and final output, the heaviest human organs (e.g., lung, liver) are only a few kilograms and occupy volumes far less than a cubic meter. If smaller scaffolded tissues like aortas, blood and lymph vessels, smaller bone pieces, joints, tendons, cosmetic implants, biochips, and other items are more do-able in space (e.g., due to the problem of cell mixing on earth), there will one day be a thriving market for them. “So. What’s stopping us?” ask the space enthusiasts, growing old and gray, once dazzled by sci-fi in lieu of the lunar bases, missions to Jupiter, spinning orbital stations promised by the Year 2000.
The first hurdles to successful bio-3DP in space are empirical: the failures, glitches, and SNAFUs both expected and unexpected. These are things only confrontable by cool-headed humans, immersed in their full complexity. Although extrusion devices still extrude, inkjets jet, and lasers beam just fine in microgravity, all these technologies are presently calibrated with settings for a 1G environment, where printed materials settle and can stack, and where droplets fall into their prescribed domains. In space, flow distances and rates, droplet sizes, and fluid surface tensions all exhibit radically different behaviors. Sub-millimeter-sized bubbles in fluids can be an insidious problem, as these cannot simply fizz to the surface and pop. Now, imagine a clogged fluid line in an instrument under building pressure that bursts in a lab on Earth—what a mess! (Time for a few salty phrases and a clean-up.) In contrast, zero-G messes can flow in all directions, not simply down—a potential safety hazard. Not only must many bio-3DP settings be completely reconfigured by trial-and-error, but there must be a guarantee of reliable and safe data collection both during and after in situ development in LEO. The first bioprinted tissues on the ISS with MSCs will likely thus be tiny and contained in closed systems like the ESA Microgravity Science Glovebox (MSG) or the Multiple Orbital Bioreactor with Instrumentation and Automated Sampling (MOBIAS). Obviously, full range of motion and eyeball-proximity access to bioprinted samples will be restricted over the course of bio-3DP in the near term, requiring as much prepackaging and automation as possible.
What if equipment on the high frontier needs replacing or breaks down? Space threatens to harm instruments and electronics with large temperature fluxes and ionizing radiation as much as their human custodians. Earth’s surface is “only” 400 kilometers and one firey blastoff or re-entry away. For a small jet liner, a very rough envelope calculation for a two-hour, 1,000 km flight adds up to ~$10K, or $10 per kilometer. One flight on a Falcon-9 costs approximately $67M to travel 400 vertical kilometers, or ~$168K per kilometer. Ergo, travel to LEO may be at least 10,000x more expensive than on a commercial passenger jet.
Space-based bio-3DP economics is less scary when one considers the lucrative ROI from commercial satellites, which generated $280 billion in global revenues in 2022. If there’s a job that must be done in space, humans will find a way—and profit from it handsomely. In terms of cost per kilogram, the cheapest one-way trip can be as little as $2,000. That’s well within or below the spectrum of per-kilogram cost for many high-end life sciences cell and media product units; one extreme example is the ubiquitous Taq polymerase enzyme, priced at around $100 per microgram…or a mere $100 billion per kilogram…over 1.5 million times the price of solid gold. If the potent analog of another “Taq” bio-product can only exclusively be obtained via space, one day it will be sensible to make it there, irrespective of the shipping bill. On the other hand, a major factor precluding bio-assembly lines in space is the mass load of even miniaturized machinery. Soon-to-be operational heavy lift vehicle platforms are estimated to reduce costs of 150-ton cargoes to LEO down to $100/kg and allow construction of much more voluminous shirtsleeve work environments for orbital “industrial parks.” You can surely bet that if bioengineered livers, muscle tissue, and biochips will be on those return flights, and many will contain cultured MSCs.
“Fly Me to the Moon”
This blog now concludes to bring the crucial answer to a question we’ve all been waiting for. Have MSCs been bio-printed in space? ANSWER: Yes. We are most pleased to announce that, together with the Uniformed Services University Center Biotechnology (4dBio3) and Redwire’s (formerly Techshot) Biofabrication Facility (BFF) installed hardware, RoosterBio MSCs recently flew to the International Space Station, whereupon they were printed into an artificial knee meniscus using a collagen + MSC bioink in July of this year (2023). This experiment is the very first of its kind, with others soon to follow including a demonstration of beating cardiomyocyte tissue. The ISS is not the only austere environment where RoosterBio cells have been bio-3DP’ed. A ruggedized 3D bioprinter, devised by nScrypt, Inc., was rapidly deployed to a remote location in Africa under the direction of Professor Vincent Ho of 4DBio3. It was reported in February 2023 that:
“Menisci, relatively complex forms of the cartilage, were 3D bioprinted with a gel that held their form well after printing and were then solidified slightly using a cross-linking solution. After 2 min of solidification, it was possible to remove and handle the menisci… The significance of this study provides the real possibility to 3D print ‘just-in-time’ medical solutions tailored to the need of an individual service member in any environment.”
RoosterBio MSCs were utilized both in space and in a difficult terrain/climate not merely because they are outstandingly quality controlled, easy to handle, and characterized for reliable performance. Another important feature is the uniquely high cell volume brought to bear on the bioprinting problem at hand. The 50-million Ready-to-Print (RTP)® cell vial format uses pre-expanded MSCs that can be quickly thawed, re-equilibrated to media, and mixed into a variety of bioinks for 3DP or scaled out for same-day high throughput screening (HTS). Priced in dollars per million cells, they are the cheapest MSCs commercially available. The trouble for an investigator who must wait a week or more to acquire a fully expanded of incubator filled with 50 million MSCs is thereby averted—and there’s no need to worry that one expansion of ~1M cells will be significantly different from another expanded batch. It’s a “turn-key” cell solution, all ready to go.
If all goes as planned, NASA’s Artemis II mission will depart from the Kennedy Space Center in November 2024 with four astronauts who will circle the Moon aboard the Orion spacecraft. It’s all but inevitable that audiences will tune in and hear Frank Sinatra’s classic “Fly Me to the Moon” return to lots of heavy rotation. Now here’s a bit of trivia: the late crooner barely survived a traumatic birth that permanently damaged his face and eardrum. Just maybe—somehow(?)—Sinatra’s spirit might be happy to know that space-based regenerative medicine could one day spin off breakthroughs to land back on Earth and help others with similar injuries?
References
- Nicolay, N. H., et al., Mesenchymal stem cells retain their defining stem cell characteristics after exposure to ionizing radiation. Int J Radiat Oncol Biol Phys, 2013. 87(5): p. 1171-8. 10.1016/j.ijrobp.2013.09.003
- Sutton, M. T., et al., Antimicrobial Properties of Mesenchymal Stem Cells: Therapeutic Potential for Cystic Fibrosis Infection, and Treatment. Stem Cells Int, 2016. 2016: p. 5303048. 10.1155/2016/5303048
- Giri, Jayeeta and Guido Moll, MSCs in Space: Mesenchymal Stromal Cell Therapeutics as Enabling Technology for Long-Distance Manned Space Travel. Current Stem Cell Reports, 2022. 8(1): p. 1-13. 10.1007/s40778-022-00207-y
- Pinzur, L., et al., Rescue from lethal acute radiation syndrome (ARS) with severe weight loss by secretome of intramuscularly injected human placental stromal cells. J Cachexia Sarcopenia Muscle, 2018. 9(6): p. 1079-1092. 10.1002/jcsm.12342
- Caplan, A. I. and D. Correa, The MSC: an injury drugstore. Cell Stem Cell, 2011. 9(1): p. 11-5. 10.1016/j.stem.2011.06.008
- Braid, L. R., et al., Intramuscular administration potentiates extended dwell time of mesenchymal stromal cells compared to other routes. Cytotherapy, 2018. 20(2): p. 232-244. 10.1016/j.jcyt.2017.09.013
- Huang, P., et al., Feasibility, potency, and safety of growing human mesenchymal stem cells in space for clinical application. NPJ Microgravity, 2020. 6: p. 16. 10.1038/s41526-020-0106-z
- Van Ombergen, A., et al., 3D Bioprinting in Microgravity: Opportunities, Challenges, and Possible Applications in Space. Adv Healthc Mater, 2023. 12(23): p. e2300443. 10.1002/adhm.202300443
- Rezapour Sarabi, M., A. K. Yetisen, and S. Tasoglu, Bioprinting in Microgravity. ACS Biomater Sci Eng, 2023. 9(6): p. 3074-3083. 10.1021/acsbiomaterials.3c00195
- Lee, S., et al., Cell adhesion and long-term survival of transplanted mesenchymal stem cells: a prerequisite for cell therapy. Oxid Med Cell Longev, 2015. 2015: p. 632902. 10.1155/2015/632902
- Blackstone, E., M. Morrison, and M. B. Roth, H2S induces a suspended animation-like state in mice. Science, 2005. 308(5721): p. 518. 10.1126/science.1108581
- Chan, K., J. P. Goldmark, and M. B. Roth, Suspended animation extends survival limits of Caenorhabditis elegans and Saccharomyces cerevisiae at low temperature. Mol Biol Cell, 2010. 21(13): p. 2161-71. 10.1091/mbc.e09-07-0614
- Windisch, J., et al., Bioinks for Space Missions: The Influence of Long-Term Storage of Alginate-Methylcellulose-Based Bioinks on Printability as well as Cell Viability and Function. Adv Healthc Mater, 2023. 12(23): p. e2300436. 10.1002/adhm.202300436
