As an early graduate student, I remember how excited I was to see my first glowing cells after transfecting human mesenchymal stem cells (hMSCs) with a green fluorescent protein (GFP) plasmid. I was finally doing real research!
My excitement turned to disappointment after spending hours counting cells and finding out that less than 10% of cells were GFP+. I am sure many of you have shared the frustration of transfecting primary cells with mRNA or DNA. Fast forward almost 20 years and low transfection efficiencies still remain one of the main barriers to gene-modified advanced therapies.
To address this hurdle, RoosterBio and Mirus Bio have collaborated to develop a turn-key mRNA transfection protocol.
 |
 |
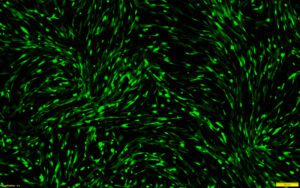
This protocol combines RoosterGEM™, a genetic engineering medium for optimized viral and non-viral applications, with Mirus’s TransIT-VirusGEN® for high-efficiency transfection of mRNA into hMSCs. Mirus developed the VirusGEN® transfection platform to increase the productivity of AAV and lentiviral production in gene and cell therapy applications. To develop the protocol, a series of collaborative optimization experiments were performed, culminating in very exciting results that were published as a poster at ASGCT [1] and ISCT earlier this year. The poster, titled “A GMP-Compatible Process for the Efficient Transfection of MSCs with mRNA,” demonstrated that > 80% transfection efficiency was achieved in hMSCs by incorporating VirusGEN® and RoosterGEM (Figure 1) into the transfection workflow.
| RoosterNourish™ | RoosterGEM™ |
|---|---|
 |
 |
 |
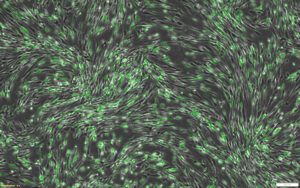 |
The bone marrow-derived hMSCs (Xeno-free RoosterVial™, RoosterBio) were cultured in either RoosterNourish™ or RoosterGEM media during transfection. Per cm2 of the culture dish, 0.2 µg of CleanCap EGFP mRNA (5-methoxyuridine, TriLink Biotechnologies) was transfected with 0.4 µl of TransIT-VirusGEN® Transfection Reagent (Mirus Bio). Representative images of transfected cells from the fluorescence (top) and brightfield-merged (bottom) channels are shown alongside the percentage of GFP+ cells measured by flow cytometry.
VirusGEN® and RoosterGEM are available in research use only (RUO) and good manufacturing practices (GMP) formulations. Therefore, they can be easily integrated into both early-stage product development and later-stage clinical manufacturing processes that require highly qualified reagents.
This protocol builds on the continuing list of applications for RoosterGEM including pDNA transfection, [2] lentiviral transduction, [3] and AAV transduction of MSCs. [4] Other emerging applications include the use of RoosterGEM to enhance electroporation, primary immune cell transduction [5], and adenovirus [6] transfection efficiencies.
How could you use RoosterGEM to improve your product development? Could you benefit from higher efficiencies and lower cost of goods by using less viral particles, mRNA, or pDNA?
I wish these technologies would have existed when I was a graduate student. They would have enabled me to do so many more interesting genetic engineering experiments with MSCs! My hope is that these protocols enable researchers spanning from beginning graduate students to experienced industry veterans to accelerate their work in ways I could not have imagined 20 years ago.
References
- Doolin, M.T., et al., Gene Editing/Gene Therapies: A GMP-COMPATIBLE PROCESS FOR THE EFFICIENT TRANSFECTION OF MSCS WITH mRNA. Cytotherapy, 2023. 25(6): p. S272.
- Doolin, M. T., T. M. Willstaedt, and J. A. Rowley, OPTIMIZATION OF TRANSIENT TRANSFECTION OF MESENCHYMAL STROMAL CELLS WITH PLASMID DNA OR MESSENGER RNA. Cytotherapy, 2022. 24(5): p. S59. 10.1016/S1465-3249(22)00196-7
- Willstaedt, T. M., et al., Development of an optimized lentiviral transduction medium and process to manufacture genetically modified MSC working cell banks. Cytotherapy, 2021. 23(5): p. S45. 10.1016/S1465324921003169
- Doolin, M., Willstaedt, T., Boychyn, M., Rowley, J. AAV Transduction of MSCs is Enhanced by Genetic Engineering Medium. 2023; Available from: https://www.roosterbio.com/resource/aav-transduction-of-mscs-is-enhanced-by-genetic-engineering-medium/.
- Doolin, M., Carson, J. RoosterGEM™ Shines on Lentiviral Transduction of T Cells. 2022; Available from: https://www.roosterbio.com/blog/roostergem-shines-on-lentiviral-transduction-of-t-cells/.
- RoosterBio. Webinar: The Role hMSCs Play in Bioengineering Solutions for Military Medicine. 2022; Available from: https://tinyurl.com/3rdeevr2.
