Listen to this Blog:
The “Terroir” of Umbilical Cord hMSCs
Notwithstanding one lager brand’s catchy marketing, one thing can be soundly argued: All champagne is sparkling wine, but not all sparkling wine is champagne. According to the 1891 Madrid Treaty and the latest EU law, champagne must come from the Champagne region, a cool terroir with moderate rainfall and well-drained and acidic soils that nourish the slow-ripening Pinot Meunier, Pinot noir, and Chardonnay grapes. Much like sparkling wines, mesenchymal stromal/stem cells (hMSCs) from human umbilical cords (hUC-MSCs) can also be derived in diverse ways. However, much like some New Year’s revelers who hope to awaken headache-free by splurging for higher quality stuff, clinical developers of advanced therapies increasingly ask how to stay out of trouble by choosing the right cellular starting materials.
Does the “terroir” of hMSCs matter? It’s strongly suspected that most (if not all) hMSCs originate from perivascular regions of bone, adipose tissue, umbilical cords, and other tissues. 1 Consistent with this anatomical depot, hMSCs may be instrumental “first responders” for vascular repair and angiogenesis, and the healing of wounds and broken bones. 2 Yet, unique therapeutic and/or bioprocess features can accompany each subtype, 3, 4, 5, 6, 7 and we at RoosterBio have written extensively on the nuances of each in our blogs. 8, 9, 10, 11
hUC-MSCs attract steeply increased interest as measured by the fraction of this cell type in newly posted global clinical trials involving hMSCs (celltrials.org). It’s not hard to imagine why. With hUC-MSCs derived from an often-discarded tissue via the 140,000,000 babies born each year, cryo-banked material ought not be in short supply. In addition, hUC-MSCs are known to exhibit more rapid and numerous population doublings than their adult-origin cousins. 12, 13 They are especially prolific producers of biologically potent exosomes and/or extracellular vesicles (EVs). 14 Finally, they’re beginning to establish a track record of safety, with promising signals of efficacy across some indications. 15
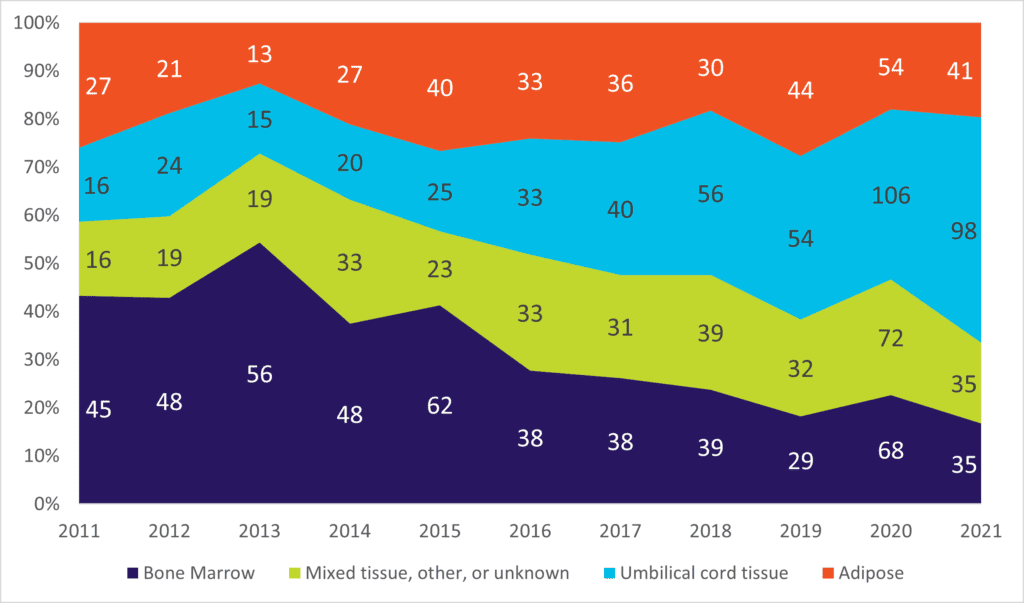
Figure 1. Distribution of different hMSC clinical trial postings by year and source of tissue, i.e., bone marrow, umbilical cord, adipose, or “other.” Note the dramatic increase in the proportion of trials involving hUC-MSCs, from ~15% in 2011 to almost half in 2021. Source: data collected from celltrials.org.
Not all hUC-MSCs are the same, however. 16 The umbilical cord is, itself, comprised of different cell types and compartments, often categorized (outside to in) by the epithelial amnion membrane (AM), the sub-amnion stroma (SA), clefts, intervascular stroma, the perivascular stroma (PV), and the blood vessels comprised of two arteries and one vein. 15, 17 The Wharton’s jelly (WJ) is the proteoglycan-rich gel substance found within the cord, intermingling with the different cell fractions. 18 Cells that resemble hMSCs and satisfy ISCT criteria can be found throughout the WJ and SA, as well as from the drained blood. 19 Nevertheless, the perivascular zones of human umbilical cord contain the highest enrichment of MSCs, and it’s proposed that some of these migrate away from PV towards the SA and beyond, acquiring different characteristics according to their new milieu across different attachment substrates and oxygen concentrations. 18 Some may even migrate into maternal lung, helping to mediate immune tolerance to the developing fetus. 20, 21
Although there are varied published methods for isolating hMSCs from cord tissue, including WJ, meticulous details of these are seldom defined in downstream in vivo or human translational studies. 4, 22 Thus, any group of random publications that involve “WJ-MSCs” could depict cells from non-transparent, haphazard techniques; some may contain PV cells, some SA cells, and some may have both. What exactly is “the hUC-MSC” being studied? Dare we ask—is your MSC biomaterial like a “champagne” or a “sparkling wine?”
HUCPVS – An Industry Standard for hUC-MSCs
If you start with different raw materials, you risk the expansion of a different cell product. Thus, it may benefit you to be a little “snobby” about where and how you get it! To reduce product heterogeneity of WJ-hMSCs, Professor John E. Davies with colleagues from U. Toronto and Tissue Regeneration Therapeutics, Inc. (TRT) devised a highly specific and novel method to obtain unique human umbilical cord perivascular cells (HUCPVs). 23 Briefly stated, HUCPVs are harvested by collecting and ligating the cord’s blood vessels into “loops,” pulling along adjacent WJ, and then digesting these loops in 50-ml conical tubes containing PBS and collagenase. After digestion, the cell suspension is immuno-depleted of CD45-containing hematopoietic cells and then plated for culture in tissue culture flasks. 2-5 million HUCPVCs can be isolated per cord, with these demonstrating a compellingly high colony-forming frequency of up to 1:333, and a million-fold expansion within 1 month’s time. 24 Such hUC-MSCs express classic MSC markers of CD105, CD73, CD90, and CD44, and are negative for STRO1, Oct4, CD43, and glycophorin A. With increased passage, they also tend to lose expression of MHC surface markers, making them especially attractive for allogeneic cell therapies. Consistent with other MSCs, HUCPVCs show inducible multipotency into bone, cartilage, adipose, and muscle tissues in vitro. Notably, HUCPVCs can be isolated from cords that had been thawed after cryopreservation in tissue banks. 25 This key feature could enable more rapid industrialization of the hUC-MSC upstream supply chain.
To reduce hUC-MSCs’ friction en route to clinical translation, RoosterBio collaborated with TRT to manufacture HUCPVCs based on TRT’s proprietary cell isolation technology and contemplated uses in medical science. 26, 27 Until recently, IP/FTO navigation related to hUC-MSC has been a challenging entry barrier for many. Today, RoosterBio’s complementary product system of perivascular hUC-MSCs, GMP and xeno-free media, and bioprocess solutions offers a clear path to all—from basic research to clinical manufacture via this unique cell type.
When we isolate hUC-MSCs in a standardized manner as per HUCPVCs, it can then be possible to define these starting materials with stringent critical quality attributes (CQAs) that will consistently govern their development as components of cellular medicines. Upstream requirements begin with the donor, who should be carefully screened before the tissue is collected according to US FDA tissue collection guidances and adherence to Good Tissue Practices (GTPs), pursuant to 21 CFR1271. 28 The tissue must be “fresh,” obtained within 24h of collection from a low-risk donor out of a full-term pregnancy via C-section. It must also test negative for a panel of pathogens and diseases including HIV, CMV, EBV, HBV, HCV, HPV, SARS-Co-V2, and other conditions. Isolation, expansion, and generation of Master Cell Banks (MCBs) and Working Cell Banks (WCBs) are ideally performed with “xeno-free” (XF) media reagents to minimize risks of zoonotic pathogen transmission and/or CJD/BSE. Finally, to assist in the creation of a Type II FDA Drug Master File (DMF) for clinical uses, the cell banks ought to be generated at a cGMP facility with full design, monitoring, and control with accompanying traceability and documentation.
No Bubbles to Burst – Premium Quality hUC-MSCs Drive Innovative Translational Research
Although the effort to obtain research-quality hUC-MSCs or HUCPVCs can be straightforward, there’s obviously a long gauntlet of testing and controls that these cells must run through before being certified as a material fit for human trials. End-to-end, across multiple pitfalls, the process can take two or more years and cost hundreds of thousands of dollars. Thankfully, however, this no longer needs to “burst the bubble” of a clinical product developer who’s interested in quickly advancing hUC-MSCs or their exosomes. Institutions such as RoosterBio have stepped up to the challenge. We can now provide these cellular tools to dedicated investigators—at a fraction of the time and cost—bypassing de novo generation of the GMP working cell bank and streamlining the regulatory process with an easily-transferrable DMF to copy into the IND filing. RoosterBio is pleased to now offer hUC-MSCs supported by licensed technology from TRT, designed for the advanced therapeutics developer, prepared cGMP and xeno-free, to support allogenic cell therapy manufacturing. 29
It’s impossible to estimate how many hUC-MSC clinical trials derive their material from Wharton’s Jelly—and of these—which actually involve cells isolated from the perivascular cells (HUCPVCs). However, I highlight here two recent investigations of special interest. First, recent work done by Professor John Campbell and colleagues at the University of Edinburgh involved GMP-compliant, xeno-free cultured HUCPVCs for co-transplantation with human pancreatic islets in preclinical studies on model diabetic mice. 30 These studies partially optimized a surgical technique and cell mixing ratio to maximize graft survival, minimize rejection, and prolong duration of restored glucose control. Compared with adipose-derived MSCs, HUCPVCs do not express inflammatory gene products in either basal or cytokine-stimulated conditions, making them an ideal MSC subtype for this therapeutic role. The authors accordingly state
“These results lay the foundation for future safety studies in animal models, which should include the tracking of MSCs by state-of-the-art molecular techniques and an assessment of long-term safety and tumorigenesis. With such data, it will be possible to proceed with randomized controlled clinical trials in man.” 30
It would be very interesting to follow this work as (one hopes) it nears readiness for clinical translation.
Another fascinating development with hUC-MSCs from Wharton’s Jelly comes to us from ENCell and Seoul’s Samsung Medical Center. Therapy against Duchenne muscular dystrophy could involve gene replacement via mini-dystrophin, but WJ-MSCs could also contribute a parallel mechanism to repair the tissue damage and fibrosis. 31 This exciting effort has led to a Phase I clinical trial that’s now recruiting patients (NCT05338099). 32 Both the U. Edinburgh and ENCell studies underscore a growing theme in MSC clinical research. That is, MSC products might be understood not merely as monotherapies, but rather as essential adjuvants to ensure the efficacy and durability of emerging cell and gene therapies.
…Now…let’s get back to that champagne. Like champagnes, not all HUCPVCs (or hUC-MSCs) need to break your budget, but particulars can matter. As in all biology (whether fruits of the vine or MSCs), context is everything. And just as a nice bottle of bubbles needs to go along with some smoky brie, witty conversation, and perhaps a little Stan Getz bossa nova, there’s certainly going to be a right time and place for hUC-MSCs. Guess what? That time just might happen to be now.
References
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C. W.; Corselli, M.; Park, T. S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; Norotte, C.; Teng, P. N.; Traas, J.; Schugar, R.; Deasy, B. M.; Badylak, S.; Buhring, H. J.; Giacobino, J. P.; Lazzari, L.; Huard, J.; Peault, B., A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3 (3), 301-13. 10.1016/j.stem.2008.07.003
- Caplan, A. I., All MSCs are pericytes? Cell Stem Cell 2008, 3 (3), 229-30. 10.1016/j.stem.2008.08.008
- Holley, R. J.; Tai, G.; Williamson, A. J.; Taylor, S.; Cain, S. A.; Richardson, S. M.; Merry, C. L.; Whetton, A. D.; Kielty, C. M.; Canfield, A. E., Comparative quantification of the surfaceome of human multipotent mesenchymal progenitor cells. Stem Cell Reports 2015, 4 (3), 473-88. 10.1016/j.stemcr.2015.01.007
- Ujiie, M.; Gomi, K.; Davies, J. In MSC functional phenotype: Assay, age and source dependence, Sci Proc, Citeseer: 2015; pp 1-5.
- Wiese, D. M.; Wood, C. A.; Ford, B. N.; Braid, L. R., Cytokine Activation Reveals Tissue-Imprinted Gene Profiles of Mesenchymal Stromal Cells. Front Immunol 2022, 13, 917790. 10.3389/fimmu.2022.917790
- Yannarelli, G.; Pacienza, N.; Cuniberti, L.; Medin, J.; Davies, J.; Keating, A., Brief report: The potential role of epigenetics on multipotent cell differentiation capacity of mesenchymal stromal cells. Stem Cells 2013, 31 (1), 215-20. 10.1002/stem.1262
- Levy, O.; Kuai, R.; Siren, E. M. J.; Bhere, D.; Milton, Y.; Nissar, N.; De Biasio, M.; Heinelt, M.; Reeve, B.; Abdi, R.; Alturki, M.; Fallatah, M.; Almalik, A.; Alhasan, A. H.; Shah, K.; Karp, J. M., Shattering barriers toward clinically meaningful MSC therapies. Sci Adv 2020, 6 (30), eaba6884. 10.1126/sciadv.aba6884
- Rowley, J. What Are MSCs? https://www.roosterbio.com/blog/what-are-mscs/ .
- RoosterBio Adipose & Bone Marrow-Derived hMSCs: What’s the Difference? https://www.roosterbio.com/blog/adipose-and-bone-marrow-derived-hmscs-whats-the-difference/ .
- Carson, J. “Where Your Eyes Don’t Go” When Considering Adipose Mesenchymal Stromal/Stem Cells. https://www.roosterbio.com/blog/where-your-eyes-dont-go-when-considering-adipose-mesenchymal-stromal-stem-cells/ .
- Carson, J. hUC-MSCs Are Unique: Are the Biomaterials, Bioprocesses, & Clinical Product Developers Now “in Accord”? https://www.roosterbio.com/blog/huc-mscs-are-unique-are-the-biomaterials-bioprocesses-clinical-product-developers-now-in-accord/ .
- Sarugaser, R.; Ennis, J.; Stanford, W. L.; Davies, J. E., Isolation, propagation, and characterization of human umbilical cord perivascular cells (HUCPVCs). Methods Mol Biol 2009, 482, 269-79. 10.1007/978-1-59745-060-7_17
- Baksh, D.; Yao, R.; Tuan, R. S., Comparison of proliferative and multilineage differentiation potential of human mesenchymal stem cells derived from umbilical cord and bone marrow. Stem Cells 2007, 25 (6), 1384-92. 10.1634/stemcells.2006-0709
- Haraszti, R. A.; Miller, R.; Stoppato, M.; Sere, Y. Y.; Coles, A.; Didiot, M. C.; Wollacott, R.; Sapp, E.; Dubuke, M. L.; Li, X.; Shaffer, S. A.; DiFiglia, M.; Wang, Y.; Aronin, N.; Khvorova, A., Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol Ther 2018, 26 (12), 2838-2847. 10.1016/j.ymthe.2018.09.015
- Chetty, S.; Yarani, R.; Swaminathan, G.; Primavera, R.; Regmi, S.; Rai, S.; Zhong, J.; Ganguly, A.; Thakor, A. S., Umbilical cord mesenchymal stromal cells-from bench to bedside. Front Cell Dev Biol 2022, 10, 1006295. 10.3389/fcell.2022.1006295
- Chen, P.; Tang, S.; Li, M.; Wang, D.; Chen, C.; Qiu, Y.; Fang, Z.; Zhang, H.; Gao, H.; Weng, H.; Hu, K.; Lin, J.; Lin, Q.; Tan, Y.; Li, S.; Chen, J.; Chen, L.; Chen, X., Single-Cell and Spatial Transcriptomics Decodes Wharton’s Jelly-Derived Mesenchymal Stem Cells Heterogeneity and a Subpopulation with Wound Repair Signatures. Adv Sci (Weinh) 2023, 10 (4), e2204786. 10.1002/advs.202204786
- Walker, J. T.; Keating, A.; Davies, J. E., Stem Cells: Umbilical Cord/Wharton’s Jelly Derived. In Cell Engineering and Regeneration, Gimble, J. M.; Marolt, D.; Oreffo, R.; Redl, H.; Wolbank, S., Eds. Springer International Publishing: Cham, 2019; pp 1-28.
- Davies, J. E.; Walker, J. T.; Keating, A., Concise Review: Wharton’s Jelly: The Rich, but Enigmatic, Source of Mesenchymal Stromal Cells. Stem Cells Transl Med 2017, 6 (7), 1620-1630. 10.1002/sctm.16-0492
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E., Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8 (4), 315-7. 10.1080/14653240600855905
- Dawe, G. S.; Tan, X. W.; Xiao, Z. C., Cell migration from baby to mother. Cell Adh Migr 2007, 1 (1), 19-27.
- Galipeau, J.; Sensebe, L., Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22 (6), 824-833. 10.1016/j.stem.2018.05.004
- Renesme, L.; Pierro, M.; Cobey, K. D.; Mital, R.; Nangle, K.; Shorr, R.; Lalu, M. M.; Thebaud, B., Definition and Characteristics of Mesenchymal Stromal Cells in Preclinical and Clinical Studies: A Scoping Review. Stem Cells Transl Med 2022, 11 (1), 44-54. 10.1093/stcltm/szab009
- Davies John, E.; Baksh, D.; Sarugaser, R.; Hosseini, M.; Lickorish Antony David, S. PROGENITOR CELLS FROM WHARTON’S JELLY OF HUMAN UMBILICAL CORD. WO 2004/072273 A1, 2004/02/10, 2004.
- Sarugaser, R.; Lickorish, D.; Baksh, D.; Hosseini, M. M.; Davies, J. E., Human umbilical cord perivascular (HUCPV) cells: a source of mesenchymal progenitors. Stem Cells 2005, 23 (2), 220-9. 10.1634/stemcells.2004-0166
- Ennis, J.; Sarugaser, R.; Davies John, E. Viable cells from frozen umbilical cord tissue. US 8278102 B2, 2006/12/21, 2012.
- RoosterBio and Tissue Regeneration Therapeutics Collaborate to Make Human Umbilical Cord-Derived MSC Bioprocess Systems Broadly Available. https://www.prweb.com/releases/roosterbio_and_tissue_regeneration_therapeutics_collaborate_to_make_human_umbilical_cord_derived_msc_bioprocess_systems_broadly_available/prweb16341107.htm .
- Farrance, I. Meeting the Growing Needs of the Perinatal RegenMed Industry: the Only Umbilical Cord hMSC System Designed for Today’s Translationally Focused Research & Product Development. https://www.roosterbio.com/blog/meeting-the-growing-needs-of-the-perinatal-regenmed-industry-the-only-umbilical-cord-hmsc-huc-msc-system-designed-for-todays-translationally-focused-research-and-product-development/ .
- FDA CFR – Code of Federal Regulations Title 21. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=1271.
- RoosterBio cGMP CliniControl™ hMSCs. https://www.roosterbio.com/products/roostervial-huc-20m-cc-c04020uc/ .
- Forbes, S.; Bond, A. R.; Thirlwell, K. L.; Burgoyne, P.; Samuel, K.; Noble, J.; Borthwick, G.; Colligan, D.; McGowan, N. W. A.; Lewis, P. S.; Fraser, A. R.; Mountford, J. C.; Carter, R. N.; Morton, N. M.; Turner, M. L.; Graham, G. J.; Campbell, J. D. M., Human umbilical cord perivascular cells improve human pancreatic islet transplant function by increasing vascularization. Sci Transl Med 2020, 12 (526). 10.1126/scitranslmed.aan5907
- Park, S. E.; Jeong, J. B.; Oh, S. J.; Kim, S. J.; Kim, H.; Choi, A.; Choi, S. J.; Oh, S. Y.; Ryu, G. H.; Lee, J.; Jeon, H. B.; Chang, J. W., Wharton’s Jelly-Derived Mesenchymal Stem Cells Reduce Fibrosis in a Mouse Model of Duchenne Muscular Dystrophy by Upregulating microRNA 499. Biomedicines 2021, 9 (9). 10.3390/biomedicines9091089
- clinicaltrials.gov Determine the Safety and Dose of EN001 in Patients With Duchenne Muscular Dystrophy(DMD). https://clinicaltrials.gov/ct2/show/NCT05338099 .
