RoosterVial™-hUC-20M-CC
cGMP xeno-free human umbilical cord-derived MSCs to support allogenic therapeutic cell manufacturing.
Designed for the advanced therapeutic developer. Fully tested and ready for cGMP manufacturing.
Recommended Products
Overview
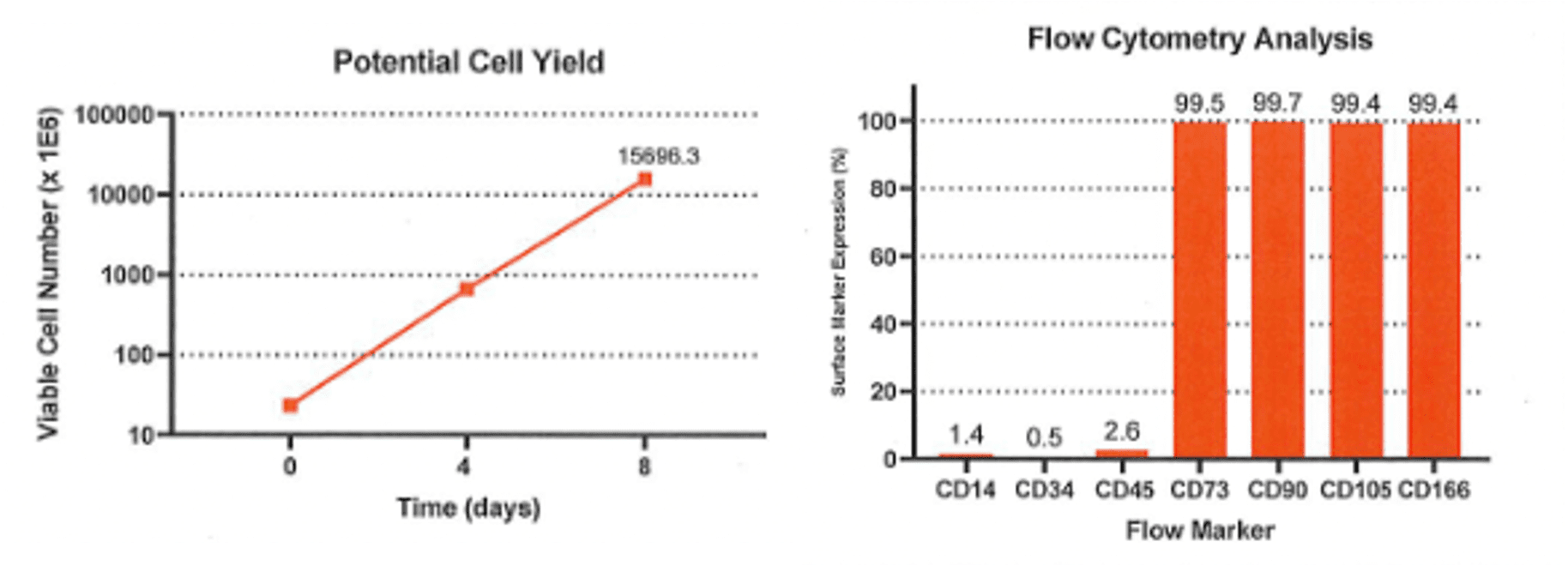
Leverage a consistent and reliable supply of our high volume, well-characterized, and standardized WCBs (working cell banks) for your large scale allogeneic cell manufacturing – and bypass major steps and risk-points in cGMP MSC production. With a rapid 2D process to generate Billions of MSCs, CliniControl™ hMSCs and expansion media get you to the clinic faster, and then scale with your clinical progression to meet your lot size requirements.
Starting with donor tissue sourced under strict guidelines, CliniControl™ (CC) human umbilical cord-derived hMSCs, isolated from the perivascular Wharton’s Jelly region of the UC, are manufactured with methods and controls that conform with current Good Manufacturing Practices (cGMP).
To further accelerate your translational research, this clinically-relevant WCB can be tested in development prior to cGMP manufacturing.
*RoosterVial-hUC MSCs are manufactured and sold by RoosterBio, INC and supported by licensed technology from Tissue Regeneration Therapeutics Inc. (TRT) core technology and patent family: US 8,790,923; US 8,278,102; US 7,547,546; US 9,611,456; US 9,611,456; US 8,481,311; US 9,611,456.
Product Features
- > 20M cryopreserved XF cells per vial
- Manufactured under GMP
- Sourced under Regional Regulatory guidelines#
- Off-the-shelf WCB – bypasses the need of sourcing tissue, qualifying and releasing your own cell banks
- Consistent manufacturing processes to reproducibly & efficiently scale to billions of cells
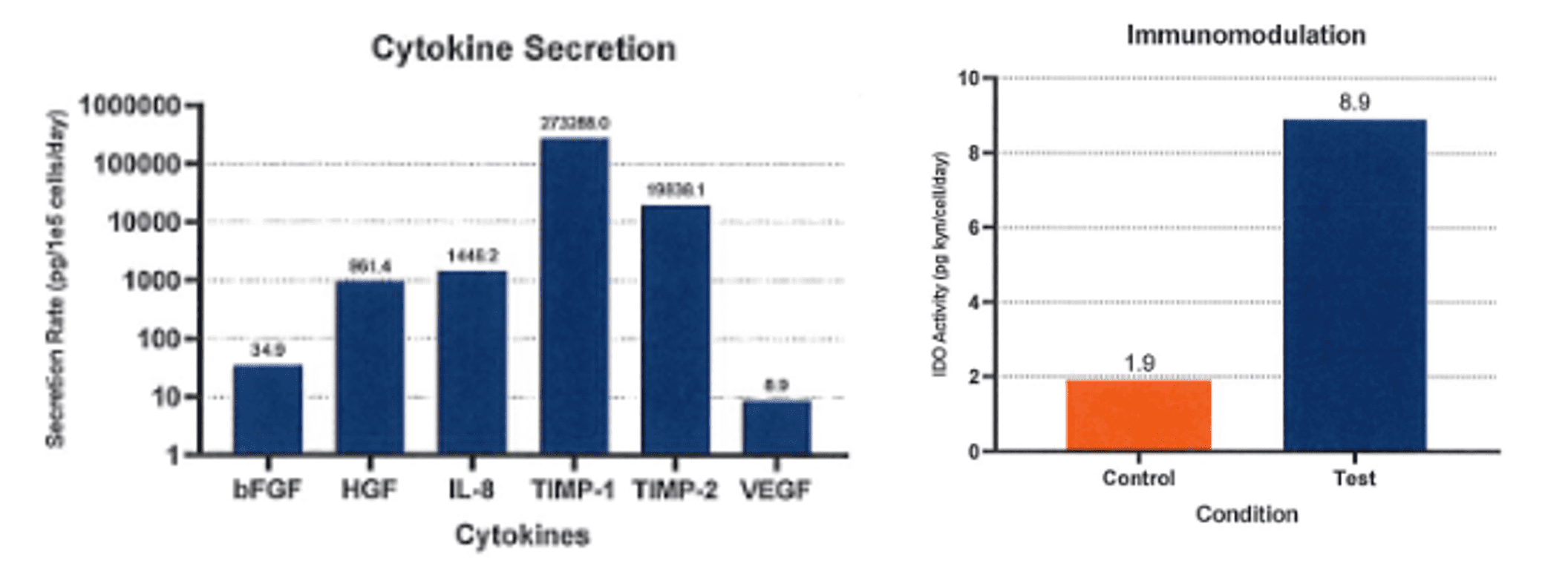
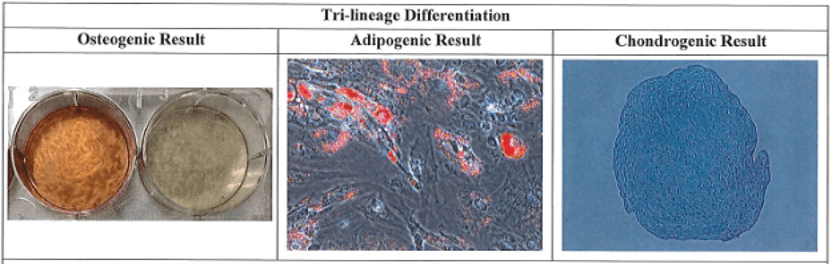
- Industry-leading functional characterization
- Tissue of Origin: Human Umbilical Cord [Wharton’s Jelly]
- Use with CliniControl™ expansion medium free from media exchange for large reduction in your Cost of Goods
- Seamless 2D (batch) to 3D (fed-batch) culture expansion
- Cryopreserved using a controlled and fully XF preservation process
Intended Use: For Further Manufacturing Use Only. Not intended for diagnostic use or for direct human or veterinary therapeutic use.
#CliniControl umbilical cord birth tissue donations comply with US FDA regulations 21 CFR Part 1271 Subparts A-C and applicable sections of Subpart D under Section 1271.150. These products are also manufactured in accordance with European Directive 2004/23/EC and 2006/17/EC. Contact us for additional region specific information.
Protocols and Information
Login to search Certificates of Analysis and Quality Control Briefs.

![RoosterVial-hUC-20M-CC-C04020UC_01[59] RoosterVial-hUC-20M-CC](https://www.roosterbio.com/wp-content/uploads/2022/11/RoosterVial-hUC-20M-CC-C04020UC_0159.png)