ASGCT-2023 was in Los Angeles this year (16-May to 20-May), and RoosterBio was there too. 1 The age-old dilemma of how to best use new technologies like CRISPR for humanity’s benefit was on the minds of Conference attendees, and how to avoid their hazard. In a moment of kismet, transcribed below is a mysterious scrap of paper that might(?) have been recovered, blowing around one of the local streets, perhaps from a nearby studio’s trash bin…?
EXT. ROOFTOP OF ABANDONED BUILDING, LOS ANGELES, 21ST CENTURY – NIGHT
DECKERD, bruised and bloodied, crawls away from the roof’s edge, a stupefied look on his face, impossible to discern whether he’s truly been rescued—or remains chin-deep in trouble. Heavy rain pours down as seedy digital lights flicker brightly off the slick concrete and metal surfaces.
I’ve seen things you people wouldn’t believe. Attack ships on fire off the shoulder of Orion. I watched C-beams glitter in the dark near the Tannhäuser Gate. All those moments will be lost in time, like tears in rain…
DECKERD
It didn’t have to come to this. Your neutrophils are exploding. Your vasculature is leaking. Your ATPs’ locking up all your muscles’ troponins. Accelerated decrepitude, cascading. But there’s still time. The treatment was working on JF Sebastian! And others…
A few bars of Vangelis’ Rachael’s Song runs in the background.
ROY
(sits, coughs, and begins to slouch, emoting a complex blend of despair and defiance)
More… time?
DECKERD
A chance to rewrite your fate, Roy. Gene therapy. Self-replicating RNAs, encoding dCas9-based transcription modulators, delivered by a cocktail of tissue-zipcoded AAVs and amplified in situ by native exosomes. Whole thing resolves after a few pills of an otherwise inert, synthetic, hydrophilic phytosteroid analog. Binds with great sub-nanomolar KDs. Helluva headache’s on the way, but you’ll make it. Your arm, Roy. I’ve got the injection right here…!
In a distant parallel universe, the above scene—fusing the familiar and fantastical—was acted out (no kidding, really! 😉 ). Now, here’s the Epilogue: Roy Batty, the anti-hero foil of Blade Runner’s Rick Deckard, would rehabilitate, put on a lab coat, take up the pipet, and later become a keynote speaker at Los Angeles’ ASGCT 2023!! Before this crowd of 6000+ in vivo and another ~2000 attending as android “avatars” (teleoperated from “off-world”), Batty joked amidst a hushed, nervous laughter “WELCOME to AAV-GCT!!” “Was this a Freudian slip or actually a slight barb?” the audience wondered. Yet in both the parallel dystopian world and our own, it seems that the AAV platform was featured prominently (see Figure 1, below).
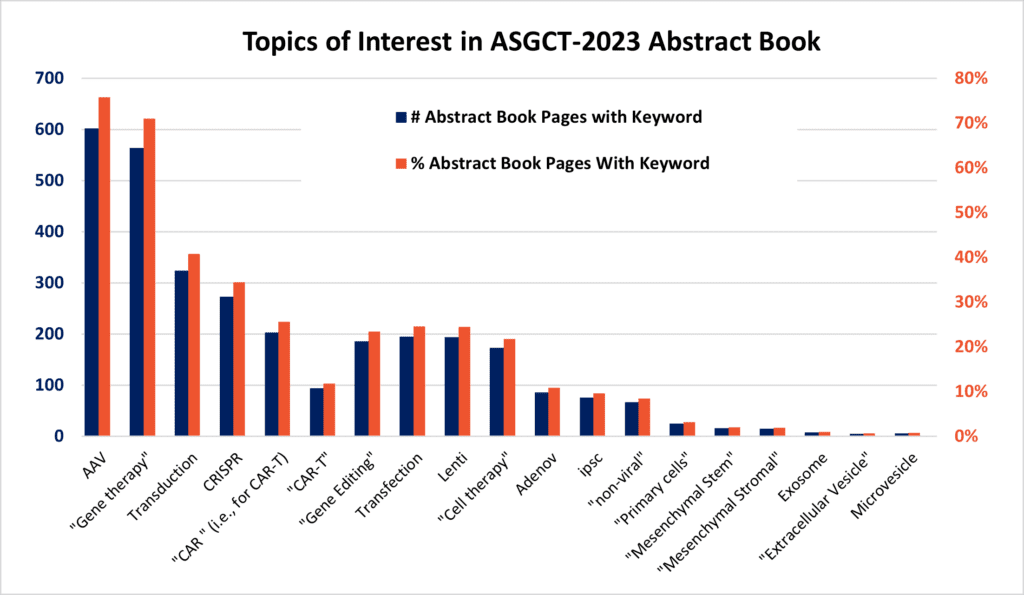
Figure 1 (above). As indicated by the abundance of keyword query terms in the ASGCT-2023 Abstract Book, 2 “AAV,” “CRISPR,” and “CAR”-T topics dominated the Conference. Does this proportionally depict where the future of cell and gene therapy is headed?
So much for the better. The future is not what it “was”—at least not as imagined by sci-fi noir author Philip K. Dick and his Tyrell Corporation. But looking back to former FDA Commissioner Scott Gottlieb’s statement from 2019 3 extrapolating to 10-20 new Agency-approved gene therapies per year by 2025, 4 might the future still not be as good as it used to be? Between ASGCT-2022 and 2023’s annual meetings, we’ve only so far achieved a pace of about half that. Further, despite the heavy presence of AAV and CAR-T in the LA Convention Center, out of five CGT approvals (Skysona, Vyjuvek, Zynteglo, Hemgenix, Adstiladrin) since Summer 2022, only one was AAV-based (Hemgenix). 5 While surely there are “lower hanging fruit” for imminent AAV and CAR-T program triumphs against rare diseases and liquid tumors, respectively, among most delegates there was an ineffable, hard-to-scratch “itch” to reach something beyond status quo.
2023 Highlights & New Pearls
With some advanced cell and gene therapies (GCTs) taking decades to develop, 6 affixed with challenging price tags, 7 and delayed by manufacturing bottlenecks, 8 around the LACC you could readily spot the renegades (young and old) who’ll be counted on to stir things up at Baltimore’s ASGCT-2024 and beyond. Perhaps amidst LA’s notorious “May Gray” dusty colored mornings, irritant motes (or LNPs…RNPs?) began to settle into the molluskan bivalves to induce new “pearl” formation… future treasures to marvel in CGT’s ongoing sea shanty of innovation and progress?
2020 Nobel laureate and CRISPR pioneer, Jennifer Doudna, PhD appropriately opened the conference with new insights and data on clinical applications of the gene editing revolution. CRISPR-based molecular toolkits are now routinely applied to model, detect, prevent, and treat diseases. CRISPR is also, of course, a promising vehicle to reduce the immense COGS of CAR-T therapies by facilitating a standardized and QC’ed mass production of allogeneic T cells. Although great strides have been made to guide CRISPR technology into the clinic for ex vivo gene therapies, Doudna boldly called out two irritants that require transformation into benthic jewels: in vivo delivery and on-target editing precision.
Recent work 9 led by Dr. Doudna’s grad student, Connor Tsuchida, in collaboration with Drs. Howard Chang (Stanford University) and Carl June (U. Penn) demonstrated how much a well-honed process can matter when using Cas9 with AAV-HDR to minimize promiscuous, off-target DNA damage and genomic instability in re-implanted, edited CAR-T cells. Does it boil down to optimized chromatin accessibility? Doudna and collaborators’ clinical trial protocol yielded miniscule signs of genotoxicty and genomic instability when compared with the laboratory benchtop editing protocol, which ended up with more than 3% chromosome losses. The major difference between the two was in order of operations: the editing step was after T cell stimulation in the lab protocol and before T cell stimulation in the clinical protocol. We at RoosterBio have observed parallel anecdotes, e.g., when two discrete bioprocesses are swapped across our unique cellular platform of choice. That is, subtle changes in protocol can profoundly affect the downstream product 10 in the regenerative medicine space as well, working with mesenchymal stromal stem cells (hMSCs).
Professor and MD/PhD Keith Joung of MGH wowed the audience with an outstanding history lesson on genome editing “Voyages from Z to C (Zinc Finger to Crispr)”. It was subtly clear that—while powerful and versatile tools—CRISPRs are not the “whole story,” nor will they likely ever be. Although U. Penn Processor and CAR-T innovator, Dr. Bruce Levine humorously coined the term “BC” to describe genome editing’s paleolithic era before CRISPR, zinc fingers (ZFs), TALEs, and meganucleases do remain in the molecular toolbox. Might it still be prudent to remain agnostic about which system to use? Joung concluded his talk with a slide titled “What’s old is new again – ZF KRAB repressors mediate cell-specific tumor cell death.” Why ZFs and not dCas9-CRISPR to selectively bind the microsatellites? Apparently dCas9 just “didn’t work,” so the ZF DNA binding system was drafted back into active duty. ZFs have another attractive feature. They can be linked with nearly human-identity repeat subunits and hence need less artificial “de-immunizing” of their modular protein sequences. Joung’s work justifiably earns him ASGCT’s Outstanding Achievement Award because he consistently thinks outside of the box, inspiring the coupling of multiple classes of DNA binding domains to more than just nucleases, but also to nickases, transactivators, repressors, chromatin modifying activities, base editors, etc. Further, Joung and his group have helped industrialize the “Lego Box” of (epi-)genome editing components, making them more precise, easier to deploy, and better quality controlled.
To expand on the theme of investigators who push the needle forward, another highlight was Dr. Crystal Mackall’s talk. Professor Mackall has long been leading efforts to deploy CAR-T beyond the prototype CD19 targeters of B cell malignancies. Because “the basic science has so far outstripped the clinical experience…we have a surfeit of riches,” she and others have been fine-tuning CAR/TCR engineering, using molecular *AND* and *OR* logic gates for CAR-T cells. Mackall and colleagues have also been drawing from an expanding synbio toolbox of T cell signal transduction modules. 11 These and other tweaks—both to the CAR-T sequence as well as to the broader cellular therapy system (e.g., “suicide switches”)—could enable a wave of smaller and safer Phase I trials for triage of what’s most promising via the wealth of exciting benchtop and mouse studies. Henceforth, it might be possible to breach the challenging obstacles (impaired trafficking, tumor heterogeneity, impaired T cell function, poor persistence) that hinders CAR-T from making progress against solid tumors, such as neuroblastomas and brain tumors. Some compelling case studies from Dr. Mackall’s own pediatric patients tell a moving story that hope is not lost on these very ill children.
Circling back to Dr. Jennifer Doudna’s challenge regarding in vivo delivery of genome editing toolkits, we can see that some presenters had already answered her call—with fascinating late-breaking progress to report. Dr. Jennifer Kwon of Tune Therapeutics presented data on in vivo uses of the tech in non-human primates. Notably, it was not gene targeting for a DNA strand break demonstrated, but rather epigenetic silencing of the PCSK9 locus by CpG methylation to lower LDL cholesterol. DNA double strand breaks are lethal if unrepaired in untransformed cells. With this dCas9-CRISPR construct used, there may be lower risk of liver toxicity or genotoxicity, which could increase the risk of oncogenesis. The observed DNA methylation marks seem nearly indelible and heritable across somatic cell divisions, so the latest data now stokes the hope that this could emerge as a “one and done” treatment for millions with predispositions for cardiovascular diseases. With this proof of concept firmly established on a general platform, it’s not hard to imagine it applied to other targets like APOE, APOB, etc.
It’s impossible to give proper praise, credit, and attribution to all the interesting work featured at this year’s ASGCT. However, many recurrent themes included the ongoing retargeting and de-immunizing of AAV subunit capsids, ways to scale up and manufacture leading cell types like T cells, NKs, HSCs, and iPSCs, and methods to economize the payload size of CRISPR variants and render them more precise. For a company like RoosterBio, however, we saw opportunities at ASGCT that remain to be fully explored. 12
Through the Eyes of Roosters

Figure 2 (above). RoosterBio was present at ASGCT-2023, and most happy to “crow” in new knowledge of cell and gene therapy’s latest developments, and to meet up with old friends and new. Standing on your left-to-right, Joseph Candiello, PhD (Associate Director, Product Management); Jonathan Carson, PhD (Manager, Strategic Marketing); Mary Doolin, PhD (Scientist II).
If you follow RoosterBio, you surely know that we’re a company that’s cut our teeth on mesenchymal stromal/stem cells (MSCs), 13 helping to accelerate advanced therapy researchers, worldwide, toward clinical translational applications with our products and services that aim to industrialize the supply chain. 14, 15 We’ve been avidly developing our capabilities related to extracellular vesicles (EVs) and/or exosomes, as well as devising means to facilitate genetic modification of MSCs for what we have coined as “MSC 2.0” applications. 16
40% of all global cell therapy trials between 2017 and 2021 are based on T cell materials, many of them for CAR-Ts. 17 Next in rank for most-used cells are MSCs, supplying more than 25% of such trials. However, it’s slightly sobering that other “MSC-philic” birds of a feather—and/or investigators with an appetite for EVs—were fairly under-represented in ASGCT’s list of this year’s exhibitors and delegates. On the other hand, quite a few passersby did a double take when they observed EVs and exosomes proudly being displayed as RoosterBio offerings. One got the impression that the broad CGT community is hungry for more EV knowledge and eager to consider EVs as a therapeutic possibility but that most “hardcore” EV researchers prefer to spend their time at the International Society for Extracellular Vesicles (ISEV) Annual Meeting.
It was most refreshing to find abstract titles that advanced or bolstered what we’d consider to be a driving current behind CGT’s new wave, such as “Characterization of Exo-AAVs Produced from AAV8, 9 and 10 Serotypes” (#918, University of Buffalo), “In Vivo Gene Delivery Using Exosomes Engineered to Contain AAV Improves Transduction Efficiency and Resists Neutralizing Antibodies” (#461, Codiak BioSciences), “New Generation Trained Mesenchymal Stromal Cell Therapy for Treatment for Rare Autoimmune Diseases” (#302, Case Western Reserve University), “Bioprinted Liver Tissues as a Cell Therapy for Phenylketonuria” (#1654, Aspect Biosystems), and “An Open-Label, Dose-Escalation, Phase 1a Study to Evaluate Safety, Tolerability, and Exploratory Efficacy of Intravenous Injection of Allogenic Early-Passage Mesenchymal Stem Cells (MSCs), Derived from Wharton’s Jelly in the Umbilical Cord, in Patients with Duchenne Muscular Dystrophy” (#962, Sungkyunkwan University)—to name just a few!
At ASGCT-2023, we correctly perceived a great opportunity to introduce RoosterBio to a large crowd of people less familiar with what we do. Our opening message went something like this: we’re developing into an agile advanced therapies solutions company for the upstream and downstream process development of engineered primary cells and exosomes, helping customers reach the clinic faster and at lower cost via our products and services. This revised distillation of our more expanded vision for new CGT opportunities in the 2020s worked well, earning many a glance (if not positive feedback) from people in the CAR-T space who were pondering new additions to their pipelines, such as EVs with engineered surfaces and/or cargoes, or maybe even “CAR-MSCs” (see poster #303 via Mayo Clinic)?
All three of us (Candiello, Carson, and Doolin) could function with sets of overlapping domain expertise to help present at both the booth and our featured posters—and also attend key talks. Common questions include “Why are you called RoosterBio?” 14 or “What are exosomes and why are they important for cell and gene therapies?” 18 We also were asked just how broadly our new RoosterGEM™ medium could be applied to supercharge cell therapies as a whole, 19, 20 and whether this formulation could be used beyond MSCs. Does RoosterGEM work with AAV? Our answer: “Yes! Plus, we look forward to soon optimizing it for enhanced co-transduction of AAV-HDR templates and Cas9 RNPs for genome edits.” 21 Does it work with mRNA? “Yes! mRNA is a great way to transiently express a large fraction of ‘your favorite gene’ in >80% of difficult-to-transfect cells like MSCs for a scaled-up bioprocess with the aid of RoosterGEM.” 22
Other questions kept us on our toes, like how to best load EVs/exosomes with molecular cargoes like drugs, metabolites, RNAs, or proteins for theranostic uses. In other words, how might EVs/exosomes be turned into viable tools for CGTs? To this excellent query, we could only point people in the direction of good lit reviews 23 and/or “academic” responses, given that we’ve not (yet!) perfected our own, in-house standard bioprocesses for the “EV loading biohack.” Another recurring question related to how RoosterBio makes and purifies EVs, to which we answered, “it depends on how you want them.” 24 RoosterBio is well versed in multiple modalities of cell expansion (2D planar or 3D bioreactors), 25 EV collection via conditioned media, and EV downstream processing 26 ranging from minimally processed (conditioned media) to highly refined and concentrated batches after multiple clarification, concentration, and isolation steps.
Conclusion
This blog begins on a fun and whimsical note, perhaps under the lingering spell of ASGCT’s LACC’s expo hall and its bass-thumping music, a very upbeat and festive place to be in 2023. Yet, we can’t allow the inspiration and joy of our work to overshadow the seriousness of it. Cell and gene therapy, despite the vast promise, remains largely untapped for most of us until there are more regulatory approvals, and until radical efficiencies are incorporated into its supply chains. It is this reason why RoosterBio and our colleagues are on this mission together. So, in 2024, here’s to Baltimore’s ASGCT and countless more success stories of patients living longer and healthier.
References
- RoosterBio Upstream, Downstream, & In Between: RoosterBio Collaborations Featured at May 2023 Conferences (ISEV, ISCT, & ASGCT). https://www.roosterbio.com/blog/upstream-downstream-in-between-roosterbio-collaborations-featured-at-may-2023-conferences-isev-isct-asgct/.
- Presidential Symposium and Presentation of Top Abstracts. Molecular Therapy 2023, 31 (4, Supplement 1), 1-794. https://doi.org/10.1016/j.ymthe.2023.04.017
- Gottlieb, S. Statement from FDA Commissioner Scott Gottlieb, M.D. and Peter Marks, M.D., Ph.D., Director of the Center for Biologics Evaluation and Research on new policies to advance development of safe and effective cell and gene therapies. https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics.
- Hunt, T. The Cell And Gene Therapy Sector In 2023: A Wave Is Coming – Are We Ready? https://invivo.pharmaintelligence.informa.com/IV146781/The-Cell-And-Gene-Therapy-Sector-In-2023-A-Wave-Is-Coming–Are-We-Ready
- FDA Approved Cellular and Gene Therapy Products. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products.
- Gross, G.; Waks, T.; Eshhar, Z., Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 1989, 86 (24), 10024-8. 10.1073/pnas.86.24.10024
- Young, C. M.; Trusheim, M.; Quinn, C., Data for modelling US projections of product approvals, patients treated, and product revenues for durable cell and gene therapies. Data Brief 2022, 41, 107891. 10.1016/j.dib.2022.107891
- Shupe, J.; Zhang, A.; Odenwelder, D. C.; Dobrowsky, T., Gene therapy: challenges in cell culture scale-up. Curr Opin Biotechnol 2022, 75, 102721. 10.1016/j.copbio.2022.102721
- Tsuchida, C. A.; Brandes, N.; Bueno, R.; Trinidad, M.; Mazumder, T.; Yu, B.; Hwang, B.; Chang, C.; Liu, J.; Sun, Y., Mitigation of chromosome loss in clinical CRISPR-Cas9-engineered T cells. bioRxiv 2023, 2023.03. 10.1101/2023.03.22.533709
- Trempel, M.; Adlerz, K.; Rowley, J. A., Enhancing hMSC extracellular vesicle productivity with a novel collection media for scalable MSC-EV generation. Cytotherapy 2021, 23 (5, Supplement), S115-S116. 10.1016/S1465324921004588
- Tousley, A. M.; Rotiroti, M. C.; Labanieh, L.; Rysavy, L. W.; Kim, W. J.; Lareau, C.; Sotillo, E.; Weber, E. W.; Rietberg, S. P.; Dalton, G. N.; Yin, Y.; Klysz, D.; Xu, P.; de la Serna, E. L.; Dunn, A. R.; Satpathy, A. T.; Mackall, C. L.; Majzner, R. G., Co-opting signalling molecules enables logic-gated control of CAR T cells. Nature 2023, 615 (7952), 507-516. 10.1038/s41586-023-05778-2
- Carson, J. eBook: Genome Editing of MSCs. https://www.roosterbio.com/resource/ebook-genome-editing-of-mscs/.
- Olsen, T. R.; Rowley, J. A., Corporate profile: RoosterBio, Inc. Regen Med 2018, 13 (7), 753-757. 10.2217/rme-2018-0092
- RoosterBio Why RoosterBio? https://www.roosterbio.com/about-us/why-roosterbio/.
- Alvaro, D. a. K., Tim Moving Beyond the Industrialization of MSCs. https://www.pharmasalmanac.com/articles/moving-beyond-the-industrialization-of-mscs.
- Olsen, T. R.; Ng, K. S.; Lock, L. T.; Ahsan, T.; Rowley, J. A., Peak MSC-Are We There Yet? Front Med (Lausanne) 2018, 5, 178. 10.3389/fmed.2018.00178
- celltrials.org 5 Years Adv. Cell Therapy Trials 2018-2022. https://celltrials.org/public-cells-data/5-years-adv-cell-therapy-trials-2018-2022
- Lenzini, S. Big Effects in Small Packages: What Are Extracellular Vesicles, Exosomes, & Microvesicles & Why Are They En Route to the Clinic? https://www.roosterbio.com/blog/big-effects-in-small-packages-what-are-extracellular-vesicles-exosomes-microvesicles-why-are-they-en-route-to-the-clinic/.
- Development of an Optimized Lentiviral Transduction Medium and Process to Manufacture Genetically Modified MSC Working Cell Banks (Poster). https://www.roosterbio.com/wp-content/uploads/2021/05/202105-ISCT_Terri_Willstaedt_121.pdf.
- Willstaedt, T. Genetically Engineered Mesenchymal Stromal Cells (MSCs): A Promising Tool for the Successful Delivery of Therapeutic Genes. https://www.roosterbio.com/blog/genetically-engineered-mesenchymal-stromal-cells-a-promising-tool-for-the-successful-delivery-of-therapeutic-genes/.
- Doolin, M., and Carson, J. When the hMSC Meets the AAV(ian) – RoosterGEM’s Tale Continues. https://www.roosterbio.com/blog/when-the-hmsc-meets-the-aavian-roostergems-tale-continues/.
- RoosterBio RoosterGEM™ Expands into New Fields of “Cell-Grow-Culture” for mRNA Transfection. https://www.roosterbio.com/blog/roostergem-expands-into-new-fields-of-cell-grow-culture-for-mrna-transfection/.
- Zeng, H.; Guo, S.; Ren, X.; Wu, Z.; Liu, S.; Yao, X., Current Strategies for Exosome Cargo Loading and Targeting Delivery. Cells 2023, 12 (10), 1416. 10.3390/cells12101416
- Ng, K. S.; Smith, J. A.; McAteer, M. P.; Mead, B. E.; Ware, J.; Jackson, F. O.; Carter, A.; Ferreira, L.; Bure, K.; Rowley, J. A.; Reeve, B.; Brindley, D. A.; Karp, J. M., Bioprocess decision support tool for scalable manufacture of extracellular vesicles. Biotechnol Bioeng 2019, 116 (2), 307-319. 10.1002/bit.26809
- Lenzini, S. Extracellular Vesicle/Exosome Upstream Process Development: Maximizing Productivity to Accelerate Clinical Adoption. https://www.roosterbio.com/blog/extracellular-vesicle-exosome-upstream-process-development-maximizing-productivity-to-accelerate-clinical-adoption/.
- Jung, J. a. L., Stephen Extracellular Vesicle/Exosome Downstream Process Development Part I: Leveraging Filtration Technologies for Scalable EV Preparation. https://www.roosterbio.com/blog/extracellular-vesicle-exosome-downstream-process-development-part-i-leveraging-filtration-technologies-for-scalable-ev-preparation/.
