Listen to this Blog:
Genetic engineering of MSCs and other primary cells is rapidly becoming transformative in the realm of cell- and extracellular vesicle-based therapies. To fuel the development of new advanced therapies, RoosterBio recently developed RoosterGEM™ genetic engineering medium. RoosterGEM has been shown to enhance heterologously expressed protein production by transduced and transfected MSCs [1, 2].
However, what if one would like to instead knock down protein production? Enter RNA interference (RNAi). Modalities such as microRNA (miRNA) or small interfering RNA (siRNA duplexes or hairpins, shRNA) can base-pair to a specific target mRNA (with a little help from the RNA-Induced Silencing Complex, RISC) to induce mRNA cleavage and/or repress expression at the level of translation. In addition to the basis for Fire and Mello’s 2006 Nobel Prize, siRNA is also the basis for four FDA-approved drugs and useful in the discovery researcher’s toolbox to dissect complex signaling pathways within MSCs. By selectively knocking down one protein at a time, that molecule’s role in clinically relevant “interactome” networks can be elucidated. Additionally, siRNA is simple to design, customize and manufacture when compared to antibodies.
siRNA has been shown to be useful in potential ex vivo modified MSC therapies for a variety of indications. For example, siRNA against IL-1β in MSCs augments their survival in vivo and enhances their regenerative potential [3]. MSCs can be innately immunosuppressive—or can be engineered to promote immune response via knockdown of iNOS [4]. Nevertheless, being primary cells, MSCs can also be quite challenging to transfect with foreign genetic material. Considering the utility and versatility of siRNA, we asked ourselves if RoosterGEM may be of assistance in delivery of a gene-silencing activity to MSCs.
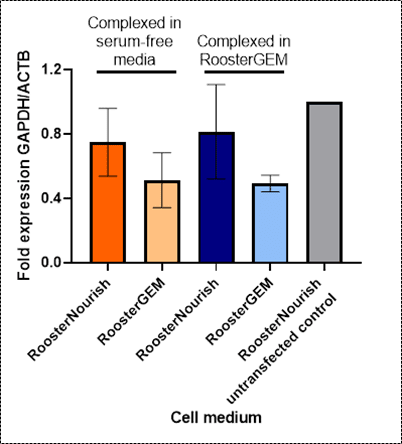 |
Figure 1. RT-qPCR results of MSCs transfected with GAPDH siRNA. Bars indicate the mean and error bars indicate standard deviation. |
To evaluate the effect of RoosterGEM on siRNA transfection, hBM-MSCs were first grown in RoosterNourish™ growth medium. On the day of transfection, cell media was changed to RoosterGEM or changed to fresh RoosterNourish. MSCs were subsequently transfected with a putative GAPDH siRNA using a commercially available lipid-based transfection reagent. siRNA-liposome complexes were made in either a commercially available serum-free medium or RoosterGEM. Media was changed back to RoosterNourish 24 hours following transfection, and GAPDH and ACTB expression were analyzed using RT-qPCR 48 hours following transfection.
Transfection in RoosterGEM enhanced the silencing of the GAPDH target transcript compared to RoosterNourish (50% inhibited vs 20% inhibited, Figure 1). RoosterGEM was also successfully able to function as the complexation milieu, eliminating the need for a third medium product (Figure 1). Naturally, a follow-up step would be to show similarly reduced expression of RNAi targets at the protein level.
This work has implications to improve MSC-based discovery research as well as ex vivo-modified therapies, as RoosterGEM makes siRNA-mediated knockdown more efficient, and therefore, the intended therapeutic impact of the knockdown may be greater. Potential targets may include cell membrane proteins that either trigger or repress an immune response, based on the indication. Perhaps, a manufacturing-favorable target would be a protein which downregulates extracellular vesicle (EV) production. With the recent advancements in siRNA modifications, siRNA can persist for longer and effects are sustainable across a bioproduction scheme. Accordingly, RoosterGEM is now available in GMP-compliant format with no licensing strings attached, and so is ready to improve your siRNA workflow whenever you are ready!
References
- Doolin, M. and Carson, J., When the hMSC Meets the AAV(ian) – RoosterGEM’s Tale Continues. 2022; Available at https://www.roosterbio.com/blog/when-the-hmsc-meets-the-aavian-roostergems-tale-continues/
- RoosterBio, RoosterGEM™ Expands into New Fields of “Cell-Grow-Culture” for mRNA Transfection. 2022; Available at https://www.roosterbio.com/blog/roostergem-expands-into-new-fields-of-cell-grow-culture-for-mrna-transfection/
- Ma, H.; Shi, X.; Yuan, X.; Ding, Y., IL-1β siRNA adenovirus benefits liver regeneration by improving mesenchymal stem cells survival after acute liver failure. Ann Hepatol 2016, 15 (2), 260-70. 5604/16652681.1193723
- Li, W.; Ren, G.; Huang, Y.; Su, J.; Han, Y.; Li, J.; Chen, X; Cao, K.; Chen, Q.; Shou, P.; Zhang, L.; Yuan, Z.R.; Roberts, A.I.; Shi, S.; Le, A.D.; Shi, Y., Mesenchymal stem cells: a double-edged sword in regulating immune responses. Cell Death Differ 2012, 19 (9), 1505–13. 1038/cdd.2012.26
- RNAi-simplified. This figure is adapted from one by Matzke MA, Matzke AJM, CC BY 2.5, via Wikimedia Commons. https://commons.wikimedia.org/wiki/File:RNAi-simplified.png
