RoosterGEM™-CC
Complete GMP Genetic Engineering Medium for Viral & Non-Viral Applications
Engineered from the ground up for simplified, highly efficient GMP primary cell engineering.
Recommended Products
Overview
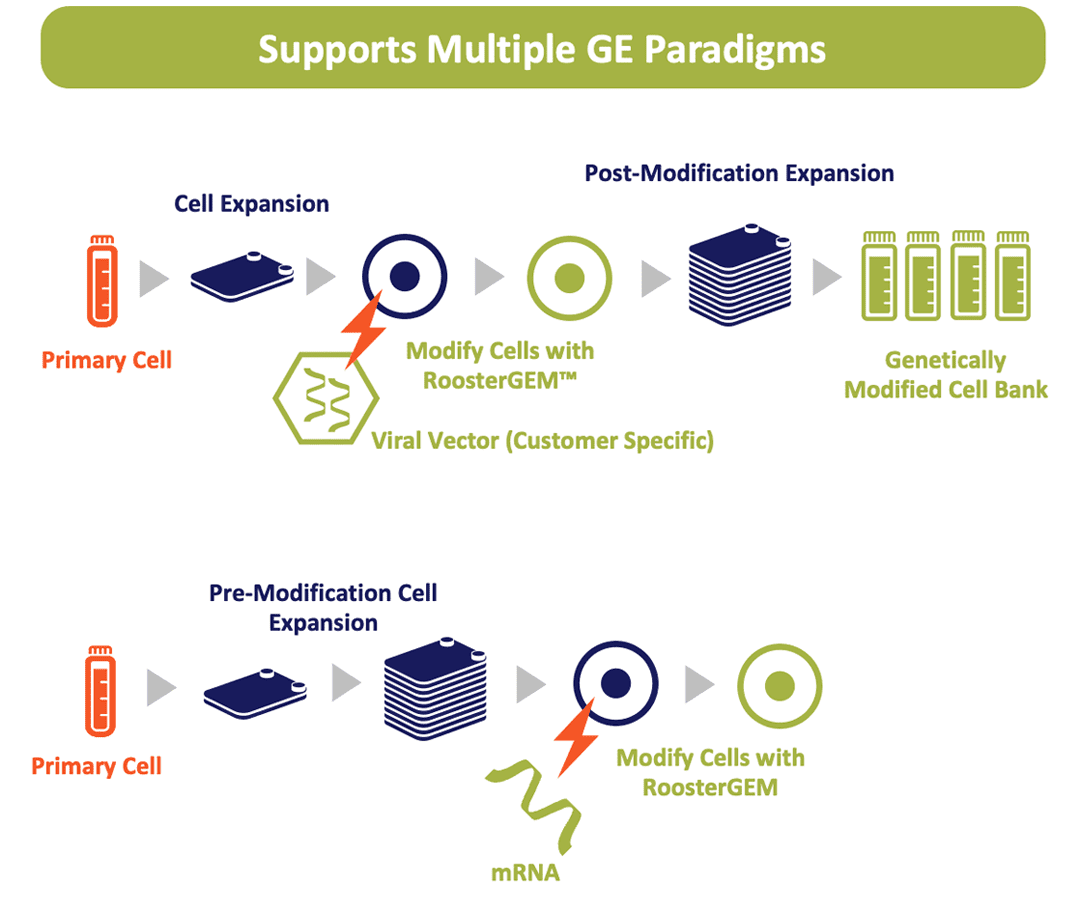
Engineered from the ground up, GMP RoosterGEM-CC is a complete media built to solve key challenges in both viral and non-viral MSC and primary cell engineering as part of a clinical cell or EV/exosome manufacturing process.
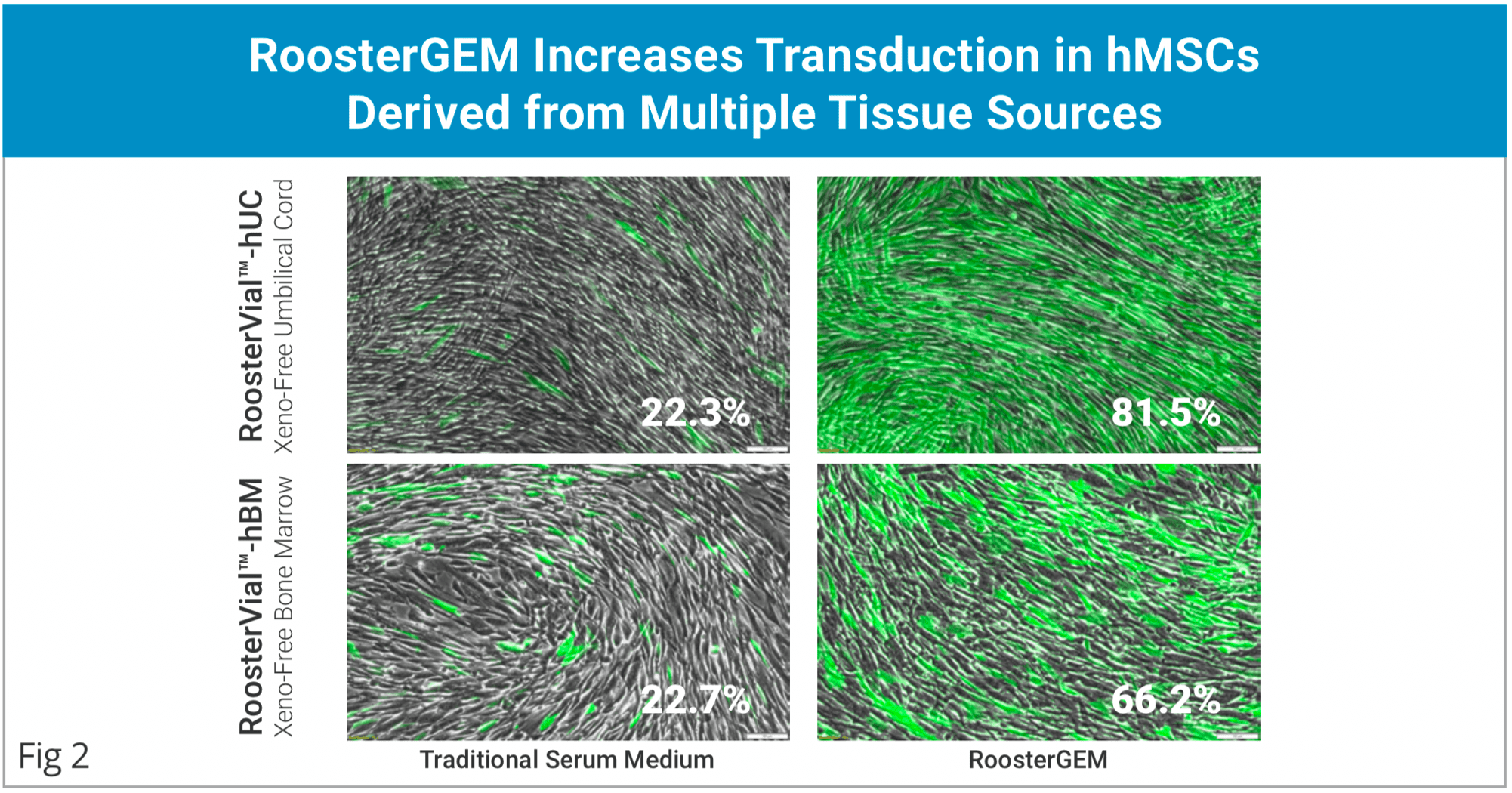
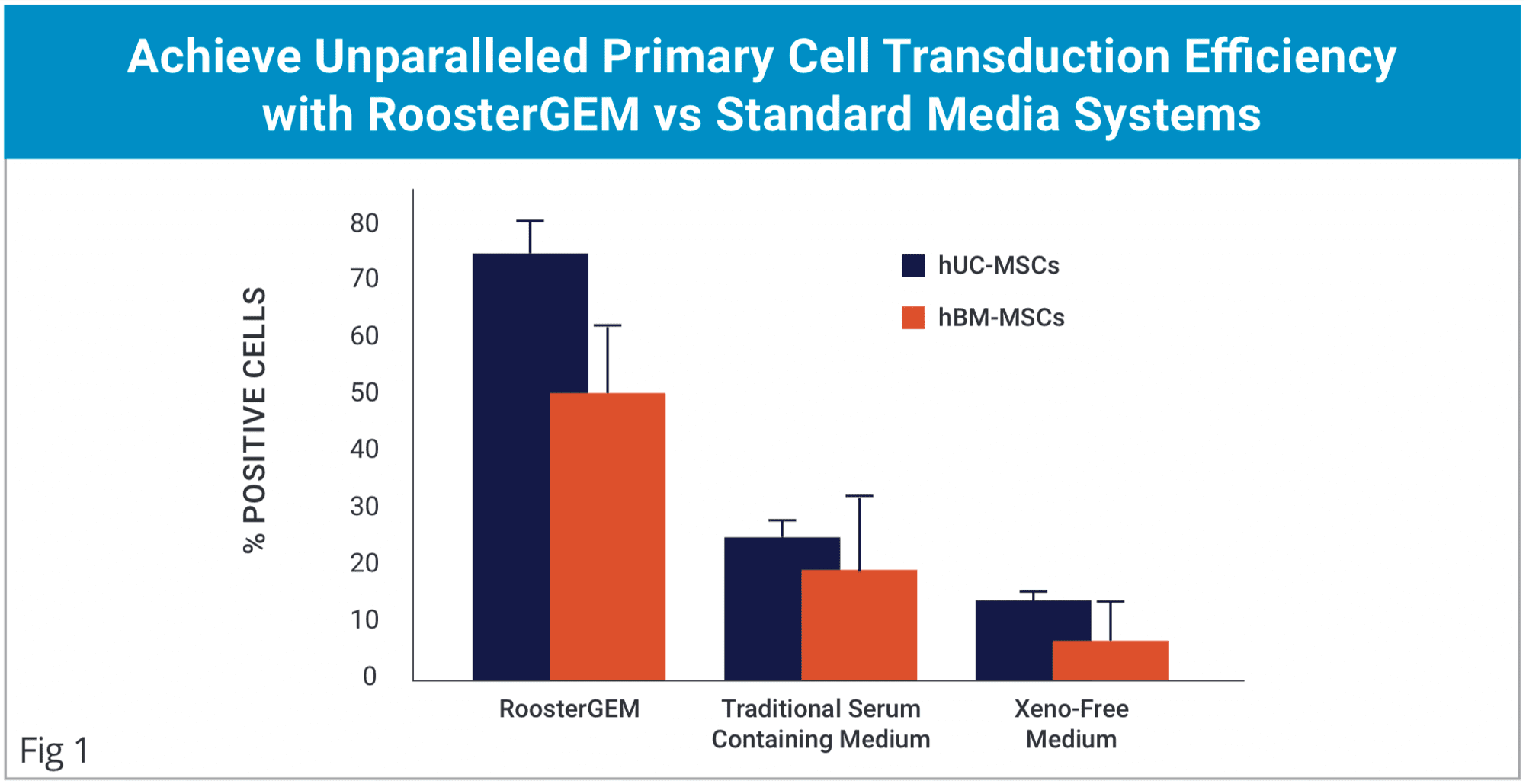
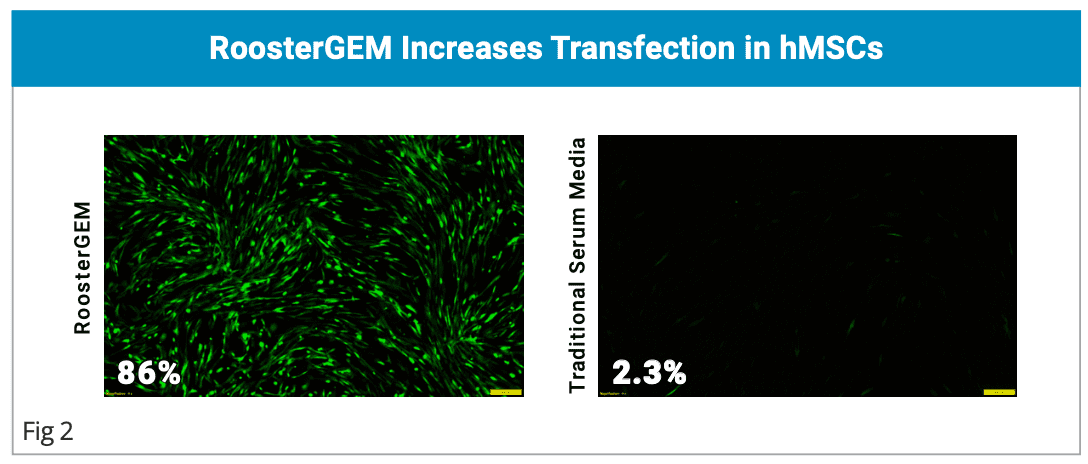
Traditionally, genetic modification of primary cells has low efficiency of genetic material transfer, requiring increased viral and non-viral agent costs, along with significant time and resources allocated to media formulation and process optimization. RoosterGEM-CC is a highly efficient GMP Complete Genetic Engineering Medium, formulated to address these key challenges in manufacturing efficiencies paired with easy to implement process recommendations.
When paired with RoosterBio’s industrialized supply chain of high-volume GMP hMSCs, ultra-productive GMP expansion media, and process development support– you enlist an end-to-end GMP Genetic Engineering MSC-platform to streamline your transient or integrated gene transfer, de-risk your product & process development, and streamline your path to clinical translation.
*RoosterGEM and RoosterGEM-CC do not require licensing for use in clinical manufacturing.
Product Features
- 200 mL GMP medium.
- Complete. Chemically defined.
- Research grade version available.
- Increased Viral and Non-viral primary cell engineering efficiency.
- Supported by simplified protocols to streamline process optimization.
- Contact RoosterBio for more information on scalable 2D or 3D bioreactor genetically modified GMP cell bank process development
*To maximize final product yield contact RoosterBio for information on low PDL cell banks.
Protocols and Information
- M40200/M03001 RoosterGEM™ mRNA Transient Transfection Recommended Protocol
- M40200/M03001 RoosterGEM™ Lentiviral Transduction Recommended Protocol
- RoosterGEM (M40200/M03001) Product Sheet
- M40220/M03001_RoosterGEM mRNA Transient transfection with VirusGEN Trans-IT
Login to search Certificates of Analysis and Quality Control Briefs.