Overview:
-
- Expansion of clinical programs to investigate hMSC extracellular vesicles, including exosomes, requires maximizing extracellular vesicle/exosome productivity in a scalable manufacturing platform.
- Extracellular vesicle/exosome productivity is determined during the upstream process by the number of cells and the number of extracellular vesicles/exosomes produced per cell.
- Rapid and efficient cell expansion facilitates a greater yield of consistent and scalable hMSC extracellular vesicle production.
- Improvements in extracellular vesicle/exosome productivity will be driven by bioreactor-based processes to amplify cell number and by media development to increase the extracellular vesicles/exosomes produced per cell.
*Unless otherwise stated, data shown in this blog was generated internally by the Process Development team at RoosterBio.
Mesenchymal stromal cells (MSCs) release lipid-bound extracellular vesicles (EVs), a broad category of tiny nano-sized particles including exosomes that mediate cell-cell communication, tissue homeostasis, and physiology. The research history and current description of extracellular vesicles/exosomes is covered in What Are Extracellular Vesicles, Exosomes, & Microvesicles, and the terms “extracellular vesicles” and “exosomes” are often used interchangeably. When delivered in vivo, human mesenchymal stem/stromal cell extracellular vesicles (hMSC-EVs) are increasingly shown to combat numerous models of disease and are currently being tested in human trials against clinical indications including kidney disease, lung disease, diabetes, and severe complications from COVID-19 infection. To learn more about hMSC-EVs in clinical trials, check out Establishing a Working Range for Effective MSC-EV Dose, where an average estimate for a generic clinical dose of extracellular vesicles was approximated at ~4×1010 hMSC-EVs. Achieving a manufacturing lot size containing hundreds or thousands of this clinical dose remains a significant challenge and requires the strategic development of a scalable bioprocess solution.
Designing a Scalable Upstream Process for Extracellular Vesicle Production
A strategy for the development of scalable hMSC extracellular vesicle manufacturing can be best understood by disassociating the approach into three distinct process arms: (i) increasing hMSC-EV production in upstream process, (ii) maximizing hMSC-EV recovery, concentration, and purity in downstream process, and (iii) providing robust, high-quality hMSC-EV standardization with characterization. We will address the upstream process in this blog post while covering downstream process and characterization in later blog posts.
Practical expansion potential for hMSC extracellular vesicle clinical trials is directly tied to reductions in the cost and risk required to manufacture extracellular vesicle doses. No fully optimized upstream process yet exists to minimize cost per hMSC-EV lot, which is estimated to be at least $1,000,000 per lot of 5×1012 hMSC-EVs [1], a size expected to cover approximately 125 clinical dose regimens, for a manufacturing cost of approximately $8000 per dose regimen. Importantly, though, increasing the sheer output of extracellular vesicles/exosomes produced in the upstream process can lead to cost savings that extend throughout the entire manufacturing process, thereby minimizing a barrier to entry for new clinical developers.
The hMSC extracellular vesicle productivity from a given upstream process shows that the total number of hMSC-EVs produced is equal to the number of cells multiplied by the number of hMSC-EVs produced per cell:
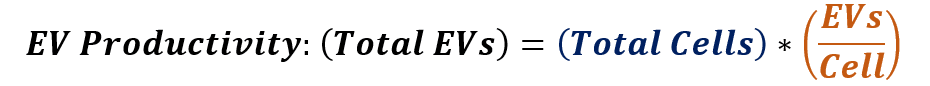
Therefore, one feasible strategy to maximize the number of hMSC-EVs generated by the upstream process is to first maximize the number of cells in the process, and then, to further increase the number of extracellular vesicles generated per cell.
Maximizing Cell Number to Maximize Extracellular Vesicle Productivity
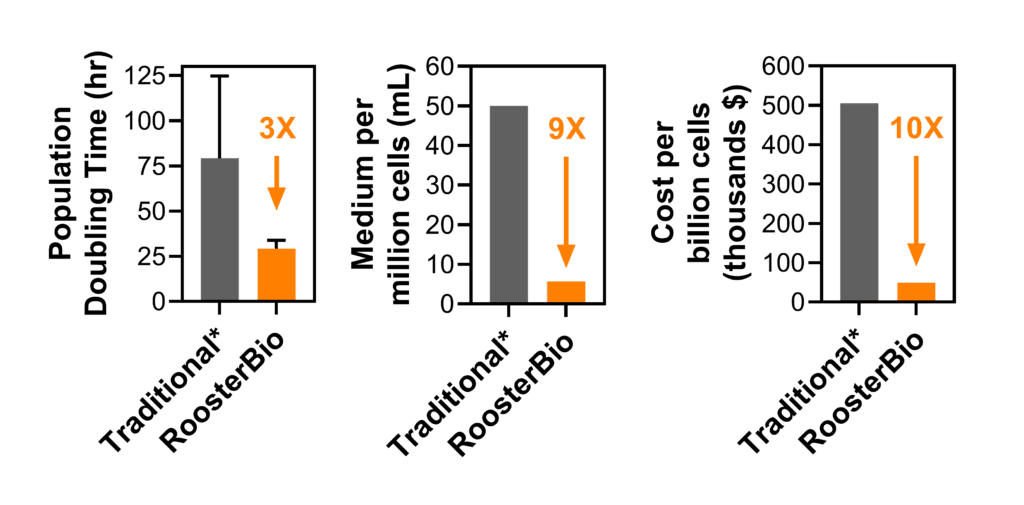
Figure 1. RoosterBio provides cells and media products that facilitate rapid cell expansion, less media consumption, and lower cost. See this Comparability of hMSC Economic & Quality Attributes blog for more information. *Data from: Siegel et. al (2013) Phenotype, donor age and gender affect function of human bone marrow-derived mesenchymal stromal cells, BMC Medicine 2013, 11:146.
Upstream process design considers extracellular vesicle/exosome production at the source: fundamentally, extracellular vesicles are produced by cells, and therefore the development of a scalable extracellular vesicle manufacturing process first demands the possibility for rapid expansion of a great number of cells. As established above, greater viable cell numbers will lead to a greater number of extracellular vesicles produced per process. Furthermore, rapid cell expansion leads to shorter process times and less medium used, with both effects driving a lower total process cost per cell produced. RoosterBio has established products and data-driven processes that yield high-quality hMSCs under xeno-free conditions at a much higher efficiency than traditional methods in terms of population doubling time, cells produced per liter of culture medium, and cost per billion cells while maintaining MSC critical quality attributes (Fig. 1). These improvements will help industrialize and right-scale the extracellular vesicle/exosome manufacturing process—not just at a reduced cost, but with reduced waste.
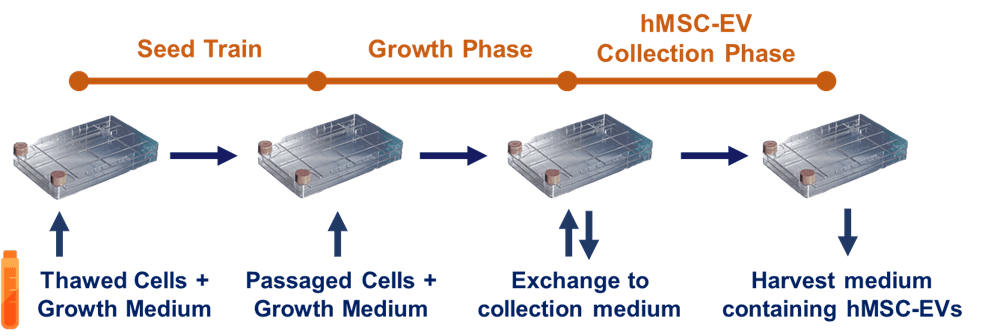
Figure 2. A generalized upstream process in extracellular vesicle/exosome manufacturing. Cells are initially expanded from a frozen bank in a seed train, followed by passaging and further expansion in growth phase. Once cells are sufficiently expanded, growth medium is exchanged to a collection medium, followed by a harvest of conditioned medium containing hMSC-EVs.
With this standard and reliable means for rapid and efficient hMSC expansion, a three-phase upstream process can then be designed for collection of extracellular vesicles/exosomes from the hMSCs. For any manufacturing process involving cells, it is important to maintain a consistent population doubling level (PDL) to ensure batch-to-batch reliability and quality. Reaching higher PDL (without passing the established upper limit) is key to maximize the number of cells obtained per batch. This is accomplished with an initial seed train for cell expansion up to a desired PDL before commencing the growth phase (to learn more about PDL, see this Population Doubling blog). Furthermore, since growth medium used for cell expansion can contain process impurities such as non-hMSC extracellular vesicles or other particles, this liquid must be discarded and washed away before the final process phase, hMSC extracellular vesicle collection, where medium is exchanged from growth medium to a collection medium. This exchange removes process impurities from the cell expansion phase. Thus, the general process strategy involves a seed train for initial cell expansion, followed by cell expansion to high density in a culture vessel, and finally, an exchange from growth medium to collection medium leading to harvest of conditioned medium (Fig. 2). Still considered a state of the art [2], this process is conventionally based on earlier academic studies that focused on the acquisition of extracellular vesicles/exosomes for analysis and characterization and/or preclinical applications.
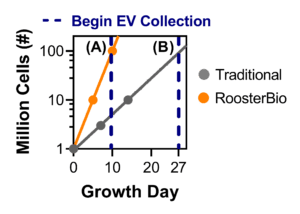 |
Figure 3. The RoosterBio advantage in hMSC expansion for hMSC-EV collection. Using RoosterBio materials, hMSCs can be expanded from 1M to 100M cells in 10 days (PDL ~6.6). Using traditional materials, hMSCs can be expanded from 1M to 3M cells in 10 days (PDL ~1.6), and 1M to 100M cells in 27 days (PDL ~ 6.6). Comparison (A) involves exchanging medium on day 10 (different PDL) and comparison (B) involves exchanging medium on day 27 (different total process time). |
|---|---|
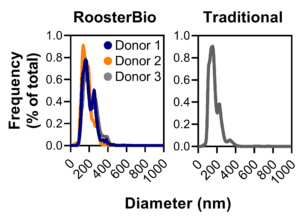 |
Figure 4. Comparison of hMSC-EV size distributions. Using RoosterBio materials, hMSC-EV size distribution is comparable across three different hMSC donors to hMSC-EVs prepared using traditional materials. Nearly all extracellular vesicles/exosomes range in diameter from 50-500 nm, with a median diameter ~180 nm. Particle size was evaluated using the Nanosight NS300 instrument, and particles were confirmed to be hMSC-EVs by evaluating proper process control samples lacking hMSCs. |
Starting with a vial of one million (1M) hMSCs, the process can obtain 100M hMSCs in 10 days using RoosterBio cells and medium (Fig. 3) with a planar flask-based culture platform. In comparison, using traditional, fetal bovine serum (FBS)-formulated cell and media materials with the same starting point requires 27 days to reach 100M hMSCs. The significantly shortened process time for the cell growth phase clearly demonstrates the efficiency of the RoosterBio platform. On day 10, the RoosterBio process reaches a post-thaw PDL ~6.6 while the traditional process reaches a post-thaw PDL~1.6. At this point, RoosterBio cell cultures can be exchanged to a collection medium. To compare appropriately to the RoosterBio process, a traditional culture can either (A) exchange medium on day 10 with a PDL ~1.6, or (B) the culture can be extended until day 27 with a medium exchange at a PDL ~6.6. Moving forward, both alternatives (A) and (B) are outlined for a thorough comparison.
Harvesting Extracellular Vesicles Using RoosterCollect™-EV
For therapeutic use, the medium used to collect hMSC extracellular vesicles should be “xeno-free” (i.e., not containing materials sourced from non-human animals) and void of contaminating particles that can leach from conventional growth medium supplements. To address this challenge, RoosterBio developed RoosterCollect-EV as a low-particulate medium to ease the implementation of the hMSC-EV collection phase (as shown in Fig. 2), leading to the accumulation of extracellular vesicle/exosomes with a consistent size distribution ranging from 50-500 nm in diameter and median ~180 nm (Fig. 4). For any given process, the collection time should be optimized to provide the maximum hMSC-EV number while maintaining critical quality attributes (CQAs) determined by characterization. Using RoosterCollect-EV in flask-based culture, this collection time is established as 3 days for both RoosterBio and traditional-sourced hMSCs, leading to a total process time of 13 days for the RoosterBio process (5-day seed train -> 5-day growth phase -> 3-day collection phase). The corresponding traditional process, then, is 13 days for alternative (A) and 30 days for alternative (B).
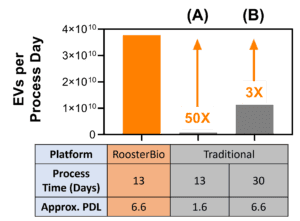 |
Figure 5. Comparison of extracellular vesicle/exosome yield using RoosterBio versus traditional materials. A process utilizing RoosterBio materials yields ~4×1010 hMSC-EVs per process day (~5×1011 total). With a constant process time (A), a similar process using traditional materials yields ~50X less EVs per process day. With a constant PDL (B), a similar process using traditional materials yields ~3X less EVs but with the requirement of 17 additional process days. |
|---|
Displayed in Fig. 5 is the hMSC extracellular vesicle yield in conditioned medium per process day when using the RoosterBio platform versus the traditional platform. As demonstrated above, the greater number of cells using the RoosterBio platform drives a greater extracellular vesicle/exosome yield, with up to a 50-fold higher yield versus traditional alternative (A). In this case, the process time is constant, clearly demonstrating the advantage of rapid and efficient cell expansion. When extending the traditional process in alternative (B) to obtain the same number of cells (and thus same PDL), the RoosterBio platform provides a ~3-fold greater yield per process day due to the significantly reduced process time. With reduced time and no media exchange between the three production phases, there is also lower potential for operator error or culture contamination. Considerations in scale-up and process cost further complicate a significantly extended process time, as labor estimates are ~$200/hour [1] and clinical development manufacturing suites cost in the range $200,000-$1,000,000 per month. This cost discrepancy will likely necessitate the compromise to a much lower PDL as evaluated in alternative (A). Thus, the RoosterBio platform provides the ability to reach a high cell number with a defined and desirable PDL via shortened process time, leading to a significantly greater yield of scalable hMSC-EV production with excellent batch-to-batch comparability.
Further Upstream Process Improvements in Bioreactors & Culture Media
After establishing the RoosterBio platform for scalable hMSC extracellular vesicle upstream process design, extracellular vesicle productivity can be further optimized. Recall that total extracellular vesicle productivity can be enhanced within an upstream process by increasing either cell density or the number of extracellular vesicles produced per cell.
Scaling up from a planar 2D flask-based system to a 3D bioreactor-based system will facilitate dramatic increases in cell density within the system. We have shown that by expanding adherent hMSCs on microcarriers in a vertical wheel [3] or with a stirred tank bioreactor system [4], hMSC density can reach greater than 500,000 cells per mL. Utilizing these systems for hMSC extracellular vesicle manufacturing provides a massive boost to extracellular vesicle productivity, primarily through increasing in-process cell density. This strategy and other discrete approaches in progress will be the subject of future blog submissions.
The Future of Scalable Extracellular Vesicle Manufacturing
RoosterBio is continuously innovating to optimize extracellular vesicle/exosome productivity and launching new products and processes that enhance the upstream process for greater feasibility of flexible and scalable extracellular vesicle manufacturing. We believe these innovations will propel clinical translation of hMSC-EVs as therapeutics and help us achieve our company’s mission. Product development at RoosterBio will continue predominantly in the spaces that will improve upstream extracellular vesicle productivity as described above: (1) scale-up in bioreactor systems that improve in-process cell density, (2) media development to enhance the extracellular vesicles/exosomes produced per cell and overall extracellular vesicle/exosome productivity. A third, more long-term direction for extracellular vesicles/exosomes is to increase their potency (in units of bioactivity per dose) through molecular engineering of their surfaces and/or intraluminal cargoes.
Given the nascent status of hMSC-EVs within biotechnology, intriguing parallels can be drawn with the growth of monoclonal antibody (mAb) bioproduction observed 20-40 years ago, where process innovations drove bioreactor productivity up ~10-fold between 1985 and 2021 to more than 3g/L using Chinese Hamster Ovary (CHO) cells. In the early mAb era (1985-2000), products were seldom approved and highly expensive to develop and produce. Today, mAb-associated cost of goods and services (COGS) have decreased significantly, yet their revenues add up to a global market of more than $100B. A similar path awaits hMSC-EV therapeutics, considering a leading indicator of related publications that exhibits exponential growth.
As described here, the upstream process is a vital component of any competitive manufacturing platform. It is thus imperative to properly characterize extracellular vesicles with the appropriate CQAs and CPPs, knowing that “Quality Begins at Inception” to standardize preparations for clinical usage. However, an optimized downstream process will further enhance the feasibility of hMSC extracellular vesicle manufacturing at an industrial scale. We thus aim to discuss downstream process and characterization methods in more depth in future blog posts.
A major objective at RoosterBio is to advance an extracellular vesicle/exosome manufacturing system complete with upstream process, downstream process, and stringent characterization that can consistently produce lots of 1013 to 1015 hMSC-EVs (~1,000 and 10,000 doses per lot, accordingly) for therapeutic purposes. This system will accommodate late-phase clinical trials and the manufacture of fully commercialized products. We invite you to join us on this journey to help make well-engineered hMSC extracellular vesicles a thriving therapeutic modality.
References
- Lembong, J., et al., Bioreactor Parameters for Microcarrier-Based Human MSC Expansion under Xeno-Free Conditions in a Vertical-Wheel System. Bioengineering (Basel), 2020. 7(3). 10.3390/bioengineering7030073
- Witwer, K.W., et al., Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J Extracell Vesicles, 2019. 8(1): p. 1609206. https://pubmed.ncbi.nlm.nih.gov/31069028/
- Ng, K. S., et al., Bioprocess decision support tool for scalable manufacture of extracellular vesicles. Biotechnol Bioeng, 2019. 116(2): p. 307-319. 10.1002/bit.26809
- Kirian, R., et al.,. Scalable MSC Suspension-Based Process Optimization using the Sartorius MSC Optimization and Characterization Solution. [Application note]. 2022. Available from: https://www.sartorius.com/en/applications/cell-and-gene-therapy/cell-therapy/cell-therapy-guide/msc-process-optimization-and-cell-product-qc-app-note
